KEY CONCEPTS
•
Superlubricity was observed for a much longer period of time when a ball coated with hydrogenated DLC was slid against a surface containing two-dimensional molybdenum disulfide and nanodiamonds.
•
The longevity of superlubricity is due to the continuous formation of onion-like carbon structures.
•
A stress-induced chemical reaction triggered by sulfur migration converted nanodiamonds into the amorphous carbon characteristic of onion-like carbon structures.
The struggle of the lubricant industry to reduce friction to as low a level as possible has moved into developing approaches to achieve superlubricity. Dr. Anirudha Sumant, a materials scientist at the Center for Nanoscale Materials, a U.S. Department of Energy Office of Science user facility at Argonne National Laboratory in Argonne, Ill., has been working on this for years. Through research efforts, his team achieved the first breakthrough in 2015, demonstrating first realization of superlubricity at macroscale, which was published in a Science article (
1).
Through detailed experimental and computation investigations, the researchers determined that graphene wrapped around nanodiamond forms nanoscrolls, which leads directly to the coefficient of friction dropping into the superlubricity range.
Since then Sumant’s efforts have been to determine how to improve it further by reducing the run-in time (time needed to reach superlubric regime) and extending the superlubricity over an extended time.
“Achieving superlubricity over a long period of time is quite challenging,” Sumant says. “We made progress in working with graphene, but there are other two-dimensional materials to evaluate. One of those materials is molybdenum disulfide.”
A well-known solid lubricant with a layered structure in its three-dimensional form, molybdenum disulfide exhibits the characteristics that may lead to superlubricity. Sumant says, “We decided to interact molybdenum disulfide with nanodiamond particles and a diamond-like carbon (DLC) surface to determine if superlubricity could be detected in a similar manner to our initial work with graphene.”
Onion-like carbon structures
Sumant and his colleagues determined that sliding a ball coating with hydrogenated DLC against a surface containing two-dimensional molybdenum disulfide and nanodiamonds produced superlubricity for a much longer period of time than when graphene was used. Figure 1a shows a schematic of the experimental setup, which was conducted using a ball-on-disk macroscale tribometer under a nitrogen atmosphere.
Sumant says, “We found that the coefficient of friction in case of the MoS
2-nanodiamond immediately reached superlubric regime within tens of cycles (
see Figure 1b) instead of a much longer running time that lasted 3,000 cycles when graphene-nanodiamond was used.” The minimum coefficient of friction detected by the researchers was 0.005 +/- 0.002.
The researchers then worked to determine how the superlubricity was achieved for such a long period of time. Sumant says, “We evaluated the wear track (
see Figure 1d) using transmission electron microscopy and time-of-flight laser desorption single photon ionization (LDSPI) and found that no molybdenum disulfide and no nanodiamonds were present. The concentration of both species declines as the number of cycles increases. This leads to the question about where did the molybdenum disulfide and nanodiamonds go?”
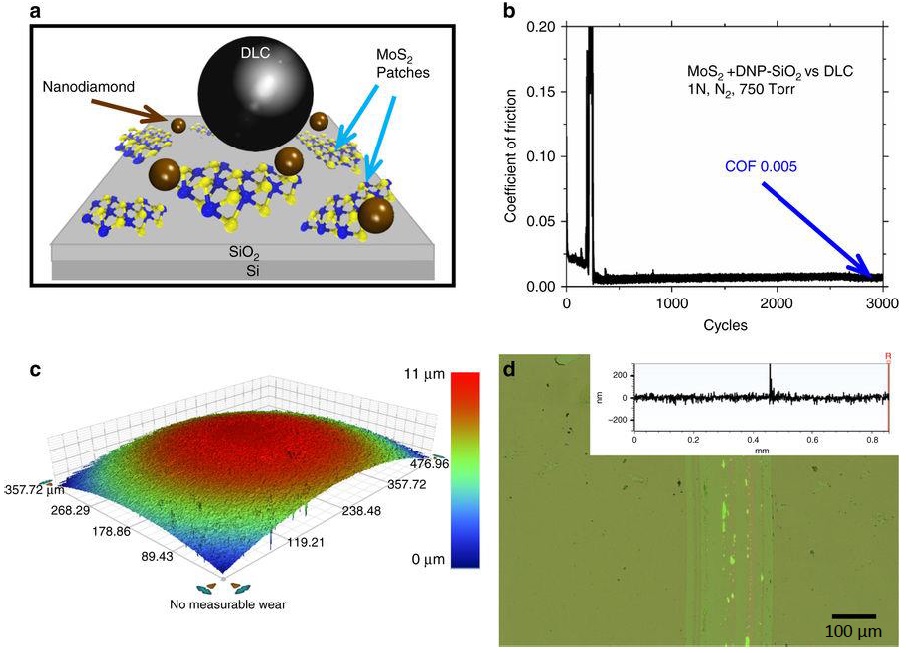 Figure 1. The experimental setup used to show superlubricity over a longer time frame is shown in (a); the coefficient of friction achieved when superlubricity was produced is shown as the experiment progresses in (b); a micrograph of the sliding ball is shown in (c); and the wear track is shown in (d). (Figure courtesy of Argonne National Laboratory.)
Figure 1. The experimental setup used to show superlubricity over a longer time frame is shown in (a); the coefficient of friction achieved when superlubricity was produced is shown as the experiment progresses in (b); a micrograph of the sliding ball is shown in (c); and the wear track is shown in (d). (Figure courtesy of Argonne National Laboratory.)
A micrograph of the sliding ball is shown in Figure 1c showing negligible wear.
The researchers discovered that onion-like carbon structures (OLCs) were produced during the process and are the source of the superlubricity. Sumant says, “LDSPI was particularly effective in establishing disintegration of molybdenum disulfide without changing the original surface composition of the wear track as it evaluates fragments of materials desorbed by the laser from the wear track.”
Due to the high contact pressure at the tribological interface, the researchers believe that molybdenum disulfide decomposes into its specific elements (molybdenum and sulfur), which enables the sulfur to diffuse onto the nanodiamonds, whereas molybdenum reacts with carbon to form molybdenum carbide. The carboxyl and oxygenated species present on the surface of the nanodiamonds bond with the sulfur atoms.
Detailed reactive molecular dynamic simulation studies led by Dr. Subramanian Sankaranarayanan, a nanoscientist at Argonne’s Center for Nanoscale Materials, revealed that sulfur migration initiates a stress-induced chemical reaction that converts the sp
3 bonded nanodiamond into sp
2 bonded carbons that are representative of the amorphous carbon. Further analysis showed that sudden increase and reduction in local temperature during each sliding cycle then convert this amorphous carbon into OLCs.
Attempts to produce superlubricity also were conducted by removing the nanodiamond leaving the molybdenum disulfide and the hydrogenated DLC coated ball and using a commercially available OLC at the interface. Neither experiment produced superlubricity.
Sumant says, “We believe that the commercially available OLC contained insufficient layers (only 5-6) to withstand the contact pressures generated when combined with molybdenum disulfide. They were worn out when we evaluated the wear track. In contrast, the OLCs generated from the interaction of molybdenum disulfide and nanodiamonds contained 30-40 layers and exhibited sufficient strength to not buckle under the empirical conditions.”
The reason for the long-lasting characteristics of superlubricity is that the OLCs are continuously being generated. Future work will involve evaluating other two-dimensional materials that may be able to produce superlubricity.
Additional information on this research can be found in a recent paper (
2) or by contacting Sumant at
sumant@anl.gov.
REFERENCES
1.
Canter, N. (2015), “Superlubricity: Seen at the macroscale for the first time,” TLT,
71 (10), pp. 10-11.
2.
Berman, D., Narayanan, B., Cherukara, M., Sankaranarayanan, S., Erdemir, A., Zinovev, A. and Sumant, A. (2018), “
Operando tribochemical formation of onion-like-carbon leads to macroscale superlubricity,”
Nature Communications,”
9, Article Number: 1164.
.