KEY CONCEPTS
•
Sodium and potassium salts of iron (II) complexes of bipyridine dicarboxylic acid and dicyanide have been developed and show promise as water soluble catholytes for redox flow batteries.
•
When hydrated, the catholyte candidates are larger than the membrane pores, which minimizes crossover to the anolyte tank improving the performance of the redox flow battery.
•
The sodium salt conducts charge under the neutral pH conditions used, which makes it the preferred salt compared to the potassium salt.
Identification of highly stable and effective energy storage devices is critical to ensure that renewable energy sources such as solar and wind can be fully optimized. One option that is under development is the redox flow battery. In a previous TLT article,
1 the components of a redox flow battery are discussed and an image shown.
A redox flow battery contains two massive electrolyte solutions known as the catholyte and the anolyte separated by an ion exchange membrane. The catholyte is situated by the cathode of the battery and contains materials that are oxidized when the battery is charging. In contrast, the anolyte is on the anode side of the battery and contains materials that are reduced. The membrane positioned between the catholyte and anolyte solutions prevents crossover of either catholytes or anolytes to avoid batery self-discharge.
Past research noted in this column has focused on non-aqueous electrolytes. A promising candidate known as CVBH,
2 derived from an analogue to a natural material, is a calcium salt of a vanadium organic complex that shows good stability in testing but is limited due to solubility in only a few non-aqueous solvents such as dimethyl sulfoxide.
The more desirable approach in producing a commercially viable redox flow battery is to use water and to prepare electrolytes that are soluble and stable in an aqueous environment. Yu Zhu, professor in the School of Polymer Science and Polymer Engineering at the University of Akron in Akron, Ohio, says, “Our objective is to develop organic electrolytes that can function in an aqueous medium in redox flow batteries. Water is safe and cheap, and we are seeking to identify electrolytes that are readily soluble and stable in water. A high degree of water solubility will enhance the energy density of the battery. Stability is a bigger challenge because electrolytes are undergoing reversible redox reactions. It is not easy to identify organic catholytes that are stable under the high oxidation voltage conditions because organic species
readily oxidize.”
Zhu and his colleagues have now synthesized and evaluated a new organic-based catholyte that shows promise as a catholyte.
Symmetry of the catholyte
The researchers developed sodium and potassium salts of iron (II) complexes of bipyridine dicarboxylic acid and dicyanide that are showing potential as aqueous catholytes in redox flow batteries. Zhu says, “These complexes solve several problems with previous aqueous catholytes. The introduction of the bipyridine derivatives introduces a bulky, water soluble ligand to iron (ii) hexacyanide. The result is the formation of asymmetric molecules that exhibit enhanced water solubility and enhanced stability. This is the highlight of our approach.”
The researchers are now able to tune the oxidation voltage of the molecule, which improves the energy density of the catholyte. Zhu adds, “Another advantage over inorganic catholytes is the increased size of the organic ligands produces charge species that cannot crossover to the anolyte tank because they are now blocked by the membrane.”
The components of the catholyte were selected based on a number of factors. Zhu says, “Iron was chosen as the transition metal because it is readily available, abundant and is readily stable during redox reaction. The bipyridine dicarboylic acid dicyanide ligands exhibit good electron withdrawal capabilities that assist with maintaining a high oxidation voltage value. They also improve the water solubility of the iron (II) complex.”
The researchers conducted a molecular dynamics analysis that measured the size of the hydrated ions of the iron (ii) complex in the catholyte. They confirmed that the hydrated catholyte iron (II) complex exhibits a larger size than the membrane pores.
Another factor that needed to be evaluated was whether the sodium salt or potassium salt of the iron (II) complex will produce better performance. Zhu says, “While potassium salts in general exhibit better water solubility than sodium salts, we decided to work with the sodium salt because the larger potassium ion does not conduct charge as rapidly under the neutral pH conditions used in our experiments.”
A redox flow battery
(see Figure 2) was prepared using the iron (II) complex as the catholyte and a well-established bipyridine derivative known as SPr-bpy as the anolyte. Both electrolytes were used at 0.1 molar concentrations, and the anolyte to catholyte electron ratio was set at 2.1:1. The catholyte reached a capacity utilization of 85% and maintained 90.5% of its initial value after 6,000 cycles.
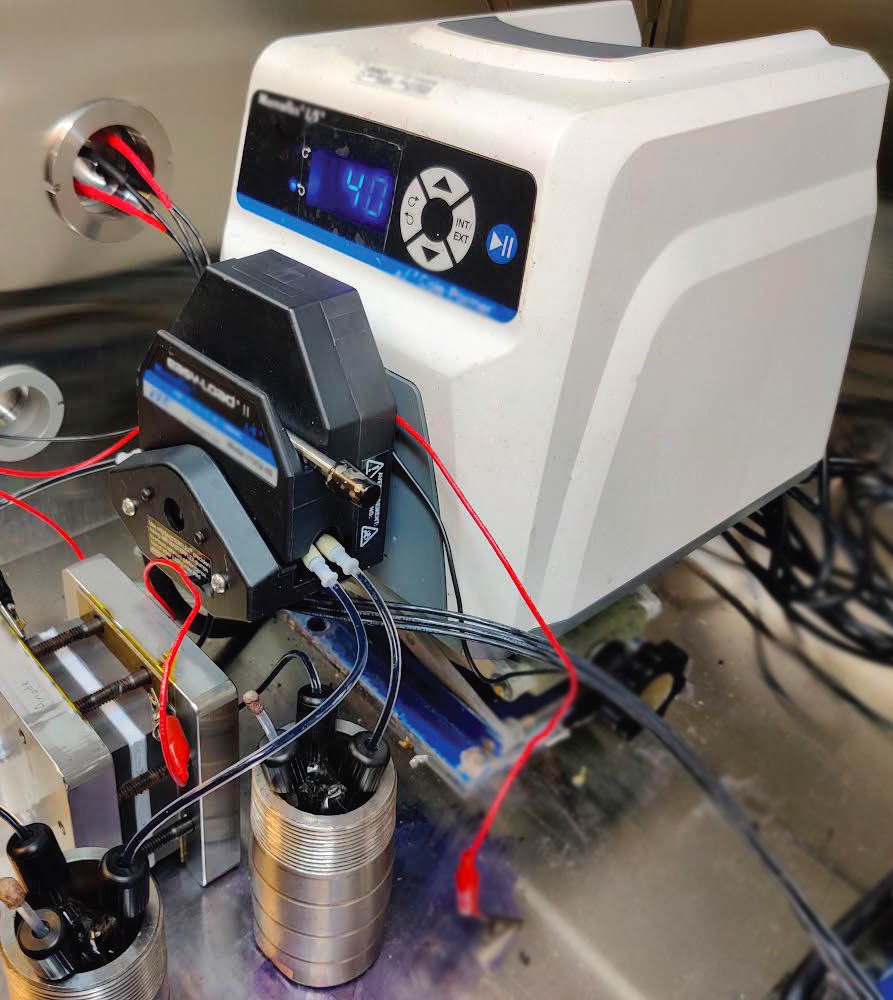 Figure 2. A water soluble organic catholyte has been introduced into the redox flow battery shown and demonstrated good performance and durability. Figure courtesy of the University of Akron.
Figure 2. A water soluble organic catholyte has been introduced into the redox flow battery shown and demonstrated good performance and durability. Figure courtesy of the University of Akron.
Zhu says, “Redox flow batteries undergo one to two cycles per day, which means that a battery with the new catholyte exhibits a stability that may last for 16 years at 90% capacity.”
The researchers are using NMR as an analysis tool to achieve two objectives. Zhu says, “NMR is very useful to understand the degree of hydration taking place between the organic catholyte and water. We also find it useful to assess the stability of the catholyte as the battery is cycling, because NMR can readily pick up potential degradation products.”
 One approach to producing a commercially viable redox flow battery is to use water and to prepare electrolytes that are soluble and stable in an aqueous environment.
One approach to producing a commercially viable redox flow battery is to use water and to prepare electrolytes that are soluble and stable in an aqueous environment.
The researchers will continue future work to identify new catholytes in an effort to improve performance and reduce cost. Zhu says, “We also will be evaluating potential organic anolytes that are compatible with the iron (II) complex. Currently, many catholytes and anolytes are operated under different pH conditions, making it difficult to identify combinations that are compatible. There are still many needs to develop electrolytes for aqueous organic redox flow batteries.”
Additional information can be found in a recent article
3 or by contacting Zhu at
yzhu@uakron.edu.
REFERENCES
1.
Canter, N. (2018), “Non-aqueous redox flow batteries,” TLT,
74 (5), pp. 20-21. Available
here.
2.
Canter, N. (2019), “Redox flow batteries: New approach may boost performance,” TLT,
75 (8), pp. 16-17. Available
here.
3.
Li, X., Gao, P., Lai, Y., Bazak, J., Hollas, A., Lin, H., Murugesan, V., Zhang, S., Cheng, C., Tung, W., Lai, Y., Feng, R., Wang, J., Wang, C., Wang, W. and Zhu, Y. (2021), “Symmetry-breaking design of an organic iron complex catholyte for a long cyclability aqueous organic redox flow battery,”
Nature Energy, 6, pp. 873-881.