A close analogue to a natural material known as Amavadin holds promise as a non-aqueous electrolyte for redox flow batteries.
The analogue known as CVBH displayed excellent stability in cyclic voltammetry and static cell testing.
Better solubility was achieved in polar solvents by reacting CVBH with other cations.
A good deal of attention has been paid to the development of batteries for use as a power source in machinery such as automobiles. This column has focused on providing updates on efforts to commercialize lithium-ion and other battery types.
The steady development of renewable and carbon-neutral energy sources such as wind and solar power has been ongoing at the same time. One challenge in working with these technologies is to figure out how to continue to supply energy when the wind is not blowing and the sun is obscured by clouds. Storing energy when excess electricity is generated for use during downtimes is a feasible option.
Redox flow batteries have been identified as a potential energy-storage device. In a previous TLT article (
1), a new type that uses a non-aqueous electrolyte was discussed. The electrolyte is based on a series of multimetallic clusters known as polyoxometalates with a hexavanadate core. In evaluation testing, the researchers found that this electrolyte enabled the redox flow battery to exhibit superior stability without a reduction in charge carrier performance.
In contrast to a lithium-ion battery, the charge-carrying species in a redox flow battery are in solution. Patrick Cappillino, assistant professor of chemistry and biochemistry at University of Massachusetts Dartmouth in Dartmouth, Mass., says, “The potential of a redox flow battery to act as a storage device is based on the ability to increase the size of battery’s storage tank to boost energy storage. This compensates for redox flow batteries having only 20% of the charge density of a lithium-ion battery but shows there is a need for improvement.”
As mentioned in the previous TLT article (
1), non-aqueous redox flow batteries (NFRB) are desired because of a limitation on the voltage potential for aqueous electrolytes. Cappillino says, “Water has a thermodynamic limit of 1.23 volts because at that point oxidation will generate oxygen creating operational problems. A similar scenario occurs below 0 volts where hydrogen is generated and occasionally needs to be bled off. Moving to a non-aqueous solvent provides much more flexibility as there are a wide variety of options to choose from including gamma valerolactone which has an electrochemical window of 8 volts, which is more than six times higher than the thermodynamic limit of water.”
Another important factor in working with a NFRB is the use of vanadium. Cappillino says, “This element is used because of its many stable oxidation states. In the operation of a redox flow battery, during charging, vanadium (3+) is converted to vanadium (2+) in one half-cell and vanadium (4+) is converted to vanadium (5+) in the second half cell.”
A new vanadium-based species inspired by a natural product has now been synthesized and shows superior stability for use in a NFRB.
CVBH
Cappillino and his colleagues have developed a new vanadium based non-aqueous electrolyte that is inspired from a natural material found in the poisonous Amanita mushrooms. The material is known as Amavadin and exhibits an extremely strong affinity to chelate vanadium for an unknown biological function.
Cappillino says, “Our interest in Amavadin originated because one of the precursors of this material is vanadyl (acac)
2 which exhibits interesting electrochemical properties and has been implemented as a NFRB active material but has experienced challenges with instability. In evaluating Amavadin, we modified a synthetic process for making a close analogue known as calcium (II) vanadium (IV) bis-hydroxyliminodiacetate (CVBH). The process is straightforward, uses inexpensive starting materials and can readily be scaled up.”
Figure 3 shows the steps taken by the researchers in developing the idea from the
Amanita mushroom, synthesizing CVBH and then evaluating it as an electrolyte in a NFRB.
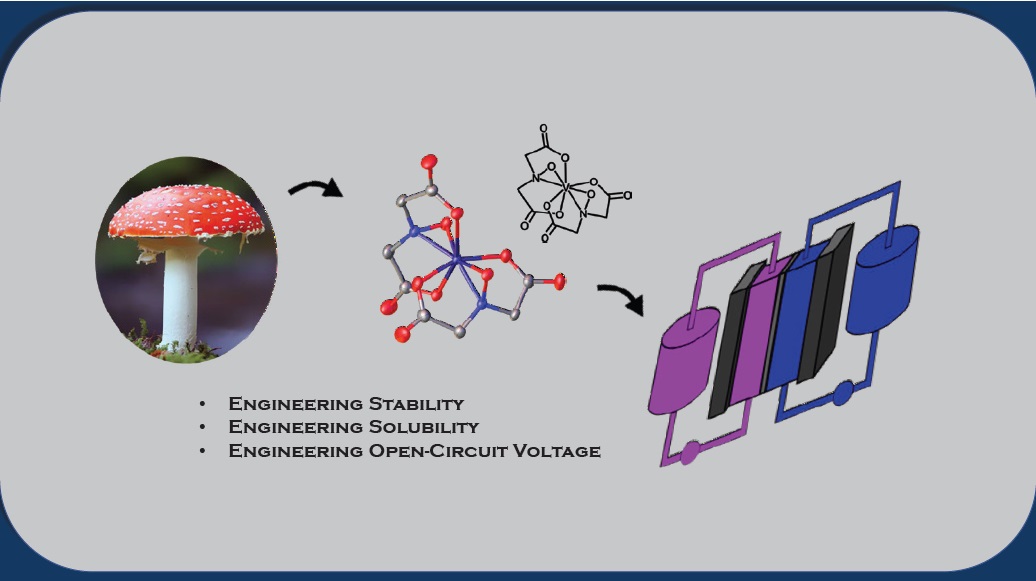 Figure 3. A new non-aqueous electrolyte that has a high affinity for vanadium was developed based on a natural material found in a mushroom and displays potential for use in redox flow batteries. (Figure courtesy of the University of Massachusetts Dartmouth.)
Figure 3. A new non-aqueous electrolyte that has a high affinity for vanadium was developed based on a natural material found in a mushroom and displays potential for use in redox flow batteries. (Figure courtesy of the University of Massachusetts Dartmouth.)
The researchers evaluated CVBH’s electrochemical properties by using cyclic voltammetry and in static cell cycling and flow cell cycling. Cappillino says, “CVBH demonstrated excellent stability in cyclic voltammetry over 800 cycles as there was no sign of a decrease in peak current and no changes in reversibility. Even the addition of 20% water (by volume) to the system did not lead to a decrease in performance.”
Cappillino noted that other vanadium-based species such as vanadyl (acac)
2 exhibit rapid hydrolysis in the presence of water. He says, “These species form vanadyl (V=O) moieties that are readily detected by analytical spectroscopy techniques. Due to their high stability, this decomposition mechanism is shut down in the mushroom-inspired compounds.”
In static cell testing, no decomposition was seen over 100 cycles and 95% conversions were achieved with bulk oxidation and bulk reduction. Flow cell cycling was conducted in collaboration with Ertan Agar, assistant professor of mechanical engineering at the University of Massachusetts Lowell in Lowell, Mass. Cappillino says, “In this process, a 1:1 mixture of vanadium (IV/V) was prepared. During discharging, vanadium (IV) was oxidized at the negative electrode while vanadium (V) was reduced at the positive electrode.”
The researchers observed that almost all of the capacity was retained after hundreds of cycles. Further analysis by UV-Vis spectroscopy indicated that the concentration of CVBH remained stable.
Cappillino says, “CVBH appears to be more stable than other vanadium-based electrolytes but its solubility is limited to only a few non-aqueous solvents such as dimethyl sulfoxide (DMSO) which was used in this study.”
To improve solubility, Cappillino’s group has reacted CVBH with other cations. He says, “We found that these new species display better solubility in polar solvents such as tetrahydrofuran and acetonitrile.”
Additional information can be found in an article (
2) published in 2017, a recent article (
3) that was just published or by contacting Cappillino at
pcappillino@umassd.edu.
REFERENCES
1.
Canter, N. (2018), “Non-aqueous Redox Flow Batteries,” TLT,
74 (5), pp. 20-21.
2.
Huang, H., Howland, R., Agar, E., Nourani, M., Golen, J. and Cappillino, P. (2017), “Bioinspired, High-stability, Nonaqueous Redox Flow Battery Electrolytes,”
Journal of Materials Chemistry A,
5 (23), pp. 11586-11591.
3.
Gokoglan, T., Pahari, S., Hamel, A, Howland R., Cappillino, P. and Agar, E. (2019), “Operando Spectroelectrochemical Characterization of a Highly Stable Bioinspired Redox Flow Battery Active Material,”
Journal of the Electrochemical Society,
166 (10), pp. A1745-A1751.