Redox flow batteries are under evaluation as energy-storage devices that can be used when power demand exceeds supply.
A series of polyoxometalates based on a hexavanadate core has been developed for use as charge carriers in non-aqueous-based redox flow batteries.
Incorporation of ethoxy groups as bridging ligands improved the stability of the polyoxometalates without a reduction in charge carrier performance.
The growing use of plug-in electric vehicles in place of internal combustion engine-powered automobiles might lead to a significant change in the vehicles consumers will be using globally in the future. An important issue that needs to be addressed is how the electric grid will be able to handle demands placed on it when many consumers wish to recharge their vehicles during the night so they are ready for use the next day.
In a previous TLT article, a simulation study was undertaken to determine how the growing use of pure electric vehicles will affect the power grid that serviced 200 households in the U.S. Midwest (
1). The conclusion is that consumers living in the same cluster who charged their plug-in electric vehicles at a fast rate stressed the electric grid to the point where it will require upgrades to the distribution infrastructure.
While this column has focused on research that may lead to the development of more cost-effective batteries for plug-in electric vehicles, concern about the electric grid also has become a research priority. One option is to use batteries as energy-storage devices that can be used when power demand exceeds available supply.
One battery type that is under evaluation is redox flow batteries (RFBs). Ellen Matson, assistant professor of chemistry at the University of Rochester in Rochester, N.Y., says, “An RFB consists of two massive tanks containing solutions of electrolyte known as the anolyte and the catholyte (
see Figure 2). During periods of charging and discharging, half of the cell is reduced and the other half is oxidized, leading the RFB to either store more power (charging cycle) or discharge the power when needed. The charging reactions in each half cell are the reverse of those that occur during the discharging period.”
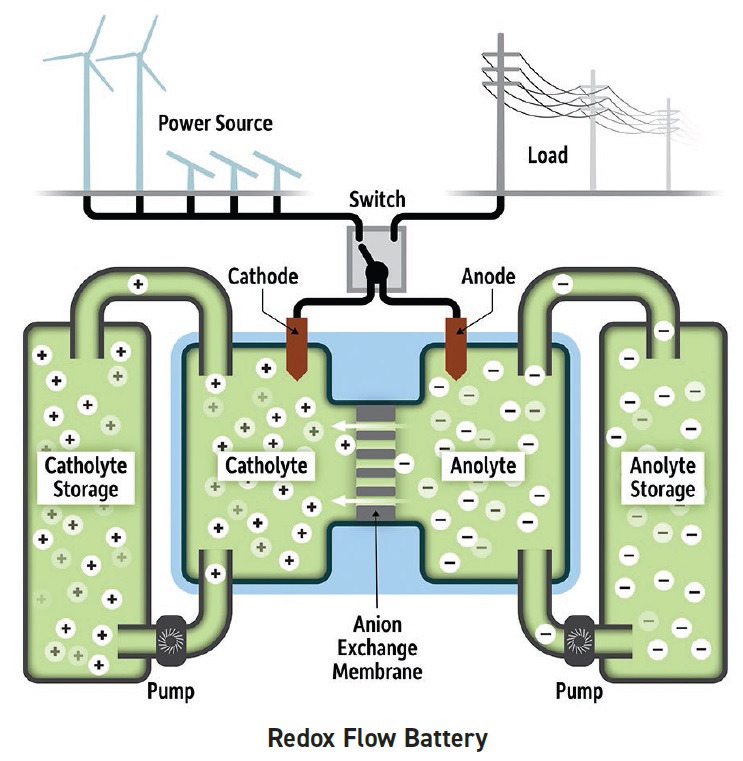 Figure 2. Development of a new charge carrier for use with a non-aqueous solvent may improve the ability of a redox flow battery to act as an energy-storage device. (Figure courtesy of the University of Rochester.)
Figure 2. Development of a new charge carrier for use with a non-aqueous solvent may improve the ability of a redox flow battery to act as an energy-storage device. (Figure courtesy of the University of Rochester.)
Four factors must be taken into consideration in identifying the right electrolyte for use in an RFB. Matson says, “The first factor is stability of the specific molecule used as the electrolyte. Any molecule used must be stable over a long time frame. Three other factors are the number of electrons transferred during the redox reactions, the concentration of the electrolyte and the voltage of the RFB cell.”
Initial developments of RFBs have focused on working with aqueous electrolytes using inorganic salts as the charge carriers. Matson says, “Aqueous-based RFBs are limited because the maximum voltage potential achieved is approximately 1.3 volts. To reach higher voltages, charge-carriers for non-aqueous redox flow batteries must be developed.”
Polyoxometalates
Matson, in collaboration with Tim Cook, assistant professor of chemistry at the University of Buffalo in Buffalo, N.Y., has developed a series of multimetallic clusters known as polyoxometalates that display potential as charge carriers in non-aqueous RFBs. She says, “Polyoxometalates contain three or more transition metal oxyanions that are bridged by alkoxide ligands to form three-dimensional structures. The presence of the alkoxide ligands enables metals to be present in the cluster in different oxidation states and minimizes solubility concerns in non-aqueous media. Without these ligands, the cluster would be totally insoluble and behave similar to a rock in water.”
Another benefit in using these polyoxometalates is that their synthesis is relatively simple as it involves the self assembly of metal centers into a well-defined structure in a high yield. The researchers worked with a hexavanadate core that combined two types of vanadium atoms in different oxidation states. The hexavanadate core was selected because of the well-established redox properties of vanadium ions.
The researchers used acetonitrile as the solvent because it has a voltage window that is nearly four times higher than water. Additionally, acetonitrile has physical properties (e.g., viscosity and boiling/freezing points) rendering this organic solvent ideal for implementation in non-aqueous RFBs.
Initially the researchers evaluated a hexavanadate polyoxometalate prepared using methoxy groups as the bridging ligands. The electrochemical properties of this cluster were evaluated using cyclic voltammetry. But long-term solution-phase stability of the various charge states of the polyoxometalate showed that this cluster was not stable under highly oxidizing conditions. When the methoxy-based polyoxometalate was added as the charge-carrier for the anolyte and catholyte in an RFB, instability was detected with the anolyte solution.
Matson says, “We then decided to substitute ethoxy groups as the bridging ligands in place of methoxy groups with the expectation that a larger positive inductive effect would minimize instability.”
The researchers found that a hexavanadate polyoxometalate with bridging ethoxy groups exhibited superior stability without a reduction in charge carrier performance. Matson says, “The one problem we now face is that the less polar ethoxy-based polyoxometalate is not as soluble in the polar solvent, acetonitrile compared to the cluster using methoxy groups as the bridging ligands.”
An additional benefit of these polyoxometalates is that crossover is minimized. Matson says, “The large size of the clusters prevents them from crossing from the anolyte side to the catholyte side. Smaller molecules that cross over can negatively impact the performance of the RFB.”
Matson indicates that the researcher’s objective is to develop a non-aqueous RFB that can be used in place of hydroelectric storage systems. She says, “Our current approach is commercially feasible but is still one order of magnitude too expensive.”
In the future, Matson and her research group are looking to develop clusters that exhibit comparable stability to the ethoxy-based polyoxometalates but with better solubility in acetonitrile. Matson says, “We have been working with symmetric RFBs using the same charge carrier in both the anolyte and the catholyte. But we will now work with asymmetric RFBs where the anolyte and the catholyte differ to find systems that perform in a superior fashion.”
Additional information can be found in a recent article (
2) or by contacting Matson at
matson@chem.rochester.edu.
REFERENCES
1.
Canter, N. (2018), “How will the growing use of plug-in electric vehicles affect the power grid?,” TLT,
74 (4), pp. 12-13.
2.
VanGelder, L., Kosswattaarachchi, A., Forrestel, P., Cook, T. and Matson, E. (2018), “Polyoxovanadate-alkoxide clusters as multi-electron charge carriers for symmetric non-aqueous redox flow batteries,”
Chemical Science,
9 (6), pp. 1692-1699.
.