HIGHLIGHTS
•
A flow-through dynamic triple-phase boundaries (FTDT) electrolytic cell was developed that efficiently reduces carbon dioxide to carbon monoxide.
•
Carbon dioxide was more readily transferred to the catalyst surface through the use of a porous electrode and by decreasing the local pressure.
•
Bicarbonate anion was found to be a critical transporter of carbon dioxide during the reaction.
Developing new approaches for converting carbon dioxide into useful derivatives is becoming an important approach for finding productive applications for this greenhouse gas. One of the strategies utilized by researchers is to figure out how to emulate the photosynthetic process of converting carbon dioxide to glucose.
In a previous TLT article,
1 researchers found that the bacterium
Azotobacter vinelandii is not only able to convert carbon dioxide to carbon monoxide but also can use the latter as an intermediate to synthesize simple hydrocarbons. An iron-protein associated, iron-sulfur cluster in a nitrogenase enzyme is responsible for facilely reducing carbon dioxide to carbon monoxide. The nitrogenase is able to employ carbon monoxide as an intermediate to produce ethylene, ethane and propane at ambient temperature. The researchers found that the hydrocarbon process is a secondary one that the bacterium does not need to grow. This approach may enable for the formation of hydrocarbons in a similar manner to the synthetic Fischer-Tropsch reaction.
Electrolysis is another strategy that can be used to reduce carbon dioxide to carbohydrates and water. Zhongwei Chen, Canada research chair in advanced materials for clean energy and professor of chemical engineering at the University of Waterloo in Waterloo, Ontario, Canada, says, “Conducting carbon dioxide electrolytic reduction reactions requires the use of very active catalysts because the kinetics of the process are slow in the laboratory. But to scale up this process for commercial use is a big challenge due to the need to develop better mass transfer, accelerated reaction rates and large-scale economic cells (electrode size over 100 square centimeters) to reduce costs.”
Development of an efficient process for reducing carbon dioxide must include other parameters besides the catalyst. Chen says, “Carbon dioxide is a gas at ambient temperature, which leads to the need for figuring out how to move this reactant to the catalyst site efficiently and then have the product move away from the site of the reaction. Factors such as carbon dioxide diffusion, adsorption and then desorption must be taken into consideration because if any of these steps is slower than the actual catalyzed reaction, it will be rate limiting.”
A good reactor design must take into consideration carbon dioxide diffusion to increase yields and reduce costs. The type of reduction reaction also is an important issue. There have been attempts to reduce carbon dioxide to hydrocarbons using electrolysis, but the simplest reaction to evaluate is the conversion to carbon dioxide. Chen says, “Carbon monoxide is an attractive target because it is an important intermediate in many chemical processes.”
Chen and his colleagues have now prepared a continuous reactor that can convert carbon dioxide to carbon monoxide with a yield that is 10 times higher than previously reported.
FTDT cell
The researchers achieved more efficient electrolysis through the development of a flow-through dynamic triple-phase boundaries (FTDT) electrolytic cell. An efficient reduction of carbon dioxide involved the movement of a gas saturated aqueous electrolyte through gas porous electrodes.
Chen says, “By increasing the porosity of the electrodes, the flow of carbon dioxide is much more effective making transfer to the catalyst surface easier. A second factor was to decrease the local pressure near the catalyst surface. By constricting the flow of the carbon dioxide saturated electrolyte, the solubility of carbon dioxide decreased facilitating its release from the aqueous phase and directing it to reach the catalyst surface more readily.”
The researchers used an
in situ electrodeposition process to prepare a thorough catalyst coating. Chen says, “Silver nanoparticles were deposited on hydrophilic carbon fabric present in the cathode. Using this technique was important because it can readily be scaled up for commercialization.”
Figure 3 shows a schematic of the FTDT cell with the cathode and the anode separated by a cation exchange membrane (CEM). The researchers found that the reaction of carbon dioxide to carbon monoxide is optimized by using two layers of hydrophilic carbon fabric and one layer of a hydrophobic venting material.
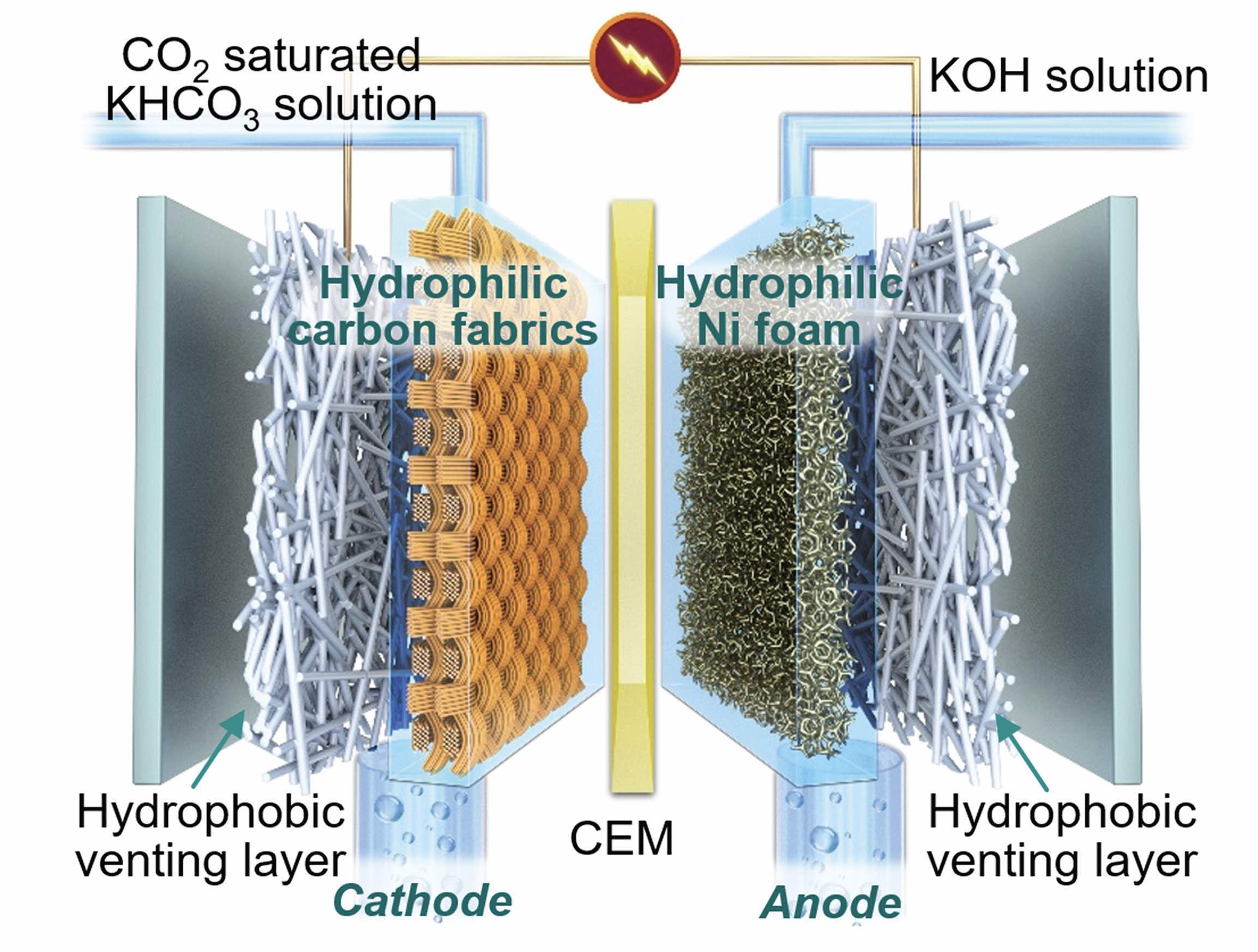 Figure 3. A schematic of the FTDT cell is shown with the key components identified. This cell effectively reduces carbon dioxide to carbon monoxide. Figure courtesy of the University of Waterloo.
Figure 3. A schematic of the FTDT cell is shown with the key components identified. This cell effectively reduces carbon dioxide to carbon monoxide. Figure courtesy of the University of Waterloo.
Testing was conducted by preparation of 4 x 100 square centimeter electrolyzer stack. A carbon monoxide flow rate of 90.6 hours per liter was realized at a maximum current density of 3.37 amperes per square centimeter.
Bicarbonate anion, which is present in the electrolyte, was found to be a critical transporter of carbon dioxide during the reaction.
In situ Raman spectral analysis showed that a band assigned to bicarbonate gradually decreases during the process, which is an indication of the conversion of this species to carbon dioxide. Chen says, “Our analysis indicates that the bicarbonate anion not only acts as a carbon dioxide transporter but also facilitates adsorption and reduction.”

The researchers are now working to scale up this process. Chen says, “We face two challenges in trying to commercialize carbon dioxide electrolysis. The lab scale process has provided good conversion of carbon dioxide to carbon monoxide for 100 hours. But for commercial purposes, the process must be effective for up to 10,000 hours. The reactor currently under evaluation is sized between the lab and the pilot plant scale. Further work needs to be done to develop a pilot plant reactor.”
The researchers also have used the electrolysis technique to convert carbon dioxide to ethylene and ethanol using a copper catalyst. Chen believes that positioning a carbon dioxide electrolysis unit in close proximity to plants using coal as a fuel can take advantage of readily available carbon dioxide and further reduce emissions and operating costs.
Additional information on this research can be found in a recent article
2 or by contacting Chen at
zhwchen@uwaterloo.ca.
1.
Canter, N. (2017), “Enzymatic conversion of carbon monoxide to hydrocarbons,” TLT,
73 (4), pp. 14-15. Available
here.
2.
Wen, G., Ren, B., Wang, X., Luo, D., Dou, H., Zheng, Y., Gao, R., Gostick, J., Yu, A. and Chen, Z. (2022), “Continuous CO
2 electrolysis using a CO
2 exsolution-induced flow cell,”
Nature Energy, 7 (10), pp. 978-988.