Enzymatic conversion of carbon monoxide to hydrocarbons
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2017
The process was run under less severe conditions than the Fischer-Tropsch reaction.
KEY CONCEPTS
•
The nitrogen-fixing bacterium,
Azotobacter vinelandii also has demonstrated the ability to reduce carbon dioxide to carbon monoxide in a detoxification process.
•
Azotobacter vinelandii has now been found to use carbon monoxide as an intermediate in the formation of simple hydrocarbons.
•
A secondary metabolic pathway is used to produce the simple hydrocarbons as a way to eliminate carbon monoxide as a potential hazard to the bacterium.
DEVELOPING BIOLOGICAL PROCESSES for converting carbon dioxide to biofuels and base oils is becoming more attractive as regulations are starting to be enacted requiring the use of more environmentally acceptable lubricants. A prime example is the Vessel General Permit (VGP) regulation that requires marine vessels operating in U.S. waters to use lubricants that meet specific biodegradability and toxicity requirements.
One strategy for developing environmentally acceptable lubricants is to utilize biological processes in simple organisms such as bacteria. A recent TLT article discussed the use of the bacterium
Clostridium ljungdahlii that converts a mixture of carbon monoxide, hydrogen and carbon dioxide into ethanol, which is widely used as a biofuel (
1). In studying this process, one challenge was to figure out how to maximize ethanol production. An important insight was that this bacterium was directed to ethanol formation if certain nutrients required for biomass production, a priority for the organism, were removed.
The process used by
Clostridium ljungdahlii is analogous to the Fischer-Tropsch reaction that chemically converts carbon monoxide and hydrogen (synthesis gas) to hydrocarbons, major components of petroleum and natural gas. Fischer-Tropsch is now being used to manufacture highly refined base oils.
Another bacterium that is under evaluation for converting carbon dioxide to hydrocarbons is
Azotobacter vinelandii. Yilin Hu, assistant professor of molecular biology & biochemistry at the Ayala School of Biological Sciences at the University of California, Irvine in Irvine, Calif., says, “Researchers have been working with
Azotobacter vinelandii for many years. This nitrogen-fixing bacterium contains a nitrogenase enzyme that enables it to convert nitrogen in the atmosphere to ammonia. The bacterium is fairly easy to work with, and the methodology used is straightforward.”
A very interesting aspect is that one component of the nitrogenase enzyme in this bacterium also can reduce carbon dioxide to carbon monoxide while the bacterium is grown under ambient, nitrogen-fixing nitrogen conditions. Markus Ribbe, professor of chemistry at the University of California, Irvine, says, “
Azotobacter vinelandii demonstrates the ability to reduce carbon dioxide to carbon monoxide, which could be part of a detoxification cascade in the cell.”
A better understanding of how this conversion takes place also led to the discovery that
Azotobacter vinelandii also can convert carbon monoxide to simple hydrocarbons.
SECONDARY METABOLIC PATHWAY
Hu, Ribbe and their coworkers have learned more about how
Azotobacter vinelandii converts carbon dioxide to carbon monoxide and discovered that this bacterium can use carbon monoxide as an intermediate to produce simple hydrocarbons.
In the first of two studies, Hu found that the key component in the nitrogenase of
Azotobacter vinelandii that is responsible for converting carbon dioxide to carbon monoxide is the iron-protein associated, iron-sulfur (Fe
4S
4) cluster. The capability of this iron protein to reduce carbon dioxide to carbon monoxide was first evaluated in an
in vitro study.
Hu says, “We used an
in vitro strategy to find a chemical approach for putting the iron-sulfur clusters into the specific redox state to convert carbon dioxide to carbon monoxide. This included using an oxidizing agent such as indigo disulfonate and a reducing agent such as dithionite to determine the proper oxidation state for the iron-sulfur clusters.”
Further analysis using electron paramagnetic resonance (EPR) was effective because the iron-sulfur clusters have free, unpaired electrons. Hu says, “EPR enabled us to determine the optimum oxidation state enabling the iron-sulfur clusters to donate electrons to facilitate the reduction of carbon dioxide.”
The researchers also determined that the reduction of carbon dioxide occurred more effectively
in vivo than
in vitro. Hu says, “We believe that the anaerobic environment of
Azotobacter vinelandii provides more ideal reducing conditions. The presence of oxygen in vitro appears to hinder the formation of carbon monoxide.”
The second study focused on evaluating the conversion of carbon monoxide to simple hydrocarbons by the vanadium nitrogenase of
Azotobacter vinelandii. Growing the bacterium at ambient temperature was initially done in the presence of ammonia to suppress nitrogen fixation and encourage biomass formation.
At this point, ammonia was removed and carbon monoxide added. The resulting nitrogenase converted carbon monoxide to ethylene, ethane and propane over an eight-hour period at ambient temperature.
Ribbe says, “But after increasing the concentration of carbon monoxide above 15% of the gas phase or doing further incubation, hydrocarbon formation plateaued. We decided to stop the process by exposing the bacterium to air, which enabled the cells to ‘relax.’ After reintroducing carbon monoxide, hydrocarbon formation resumed at a comparable rate to when the process was initiated.”
The researchers determined that hydrocarbon formation is a secondary metabolic pathway that is not required for cell growth. Ribbe says, “We propose that this pathway was used in the past by microbes when the earth’s atmosphere had a much higher concentration of carbon dioxide and is now used as a way to eliminate carbon monoxide as a potential hazard.”
Figure 3 shows a schematic model of the vanadium nitrogenase, which is the enzyme that the researchers mainly worked with in these studies.
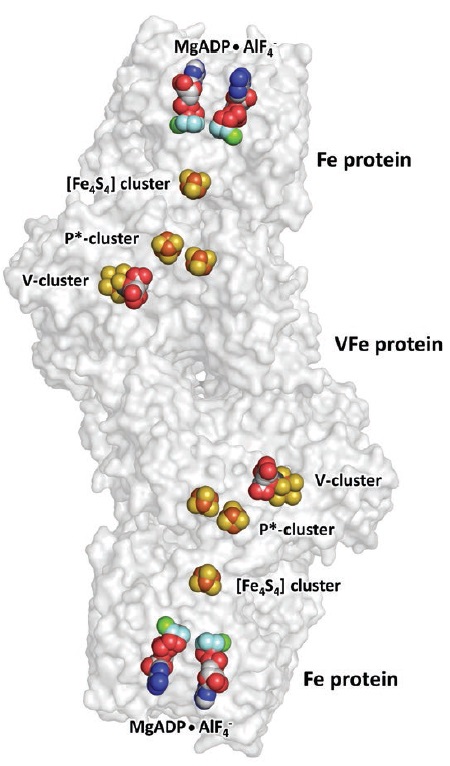 Figure 3. The vanadium nitrogenase present in the bacterium Azotobacter vinelandii is an enzyme responsible for converting carbon dioxide to simple hydrocarbons such as ethylene in a pathway where carbon monoxide is an intermediate. (Figure courtesy of the University of California, Irvine.)
Figure 3. The vanadium nitrogenase present in the bacterium Azotobacter vinelandii is an enzyme responsible for converting carbon dioxide to simple hydrocarbons such as ethylene in a pathway where carbon monoxide is an intermediate. (Figure courtesy of the University of California, Irvine.)
Ribbe says, “We believe that the
Azotobacter vinelandii process has the potential to generate hydrocarbons in an analogous manner to the Fischer-Tropsch reaction. Using the nitrogenase is an advantage because the process is run at room temperature and does not require the use of hydrogen. But our process is not as efficient and does not generate higher molecular weight hydrocarbons that could be used in fuels or lubricant base stocks.”
Future work will be focused on improving the efficiency and investigating if the enzyme can be modified to produce a single hydrocarbon instead of a mixture. Additional information can be found in references to the two studies (
2, 3) or by contacting Hu at
yilinh@uci.edu or Ribbe at
mribbe@uci.edu.
REFERENCES
1.
Canter, N. (2017), “Biofuel production using syngas fermentation,” TLT,
73 (1), pp. 14-15.
2.
Rebelein, J., Stiebritz, M., Lee, C. and Hu, Y. (2017), “Activation and reduction of carbon dioxide by nitrogenase iron proteins,”
Nature Chemical Biology,
13, pp. 147-149.
3.
Rebelein, J., Lee, C., Hu, Y. and Ribbe, M. (2016), “The in vivo hydrocarbon formation by vanadium nitrogenase follows a secondary metabolic pathway,”
Nature Communications,
7, Article # 13641.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.