The formation of a Cassie state can facilitate ice removal through the presence of trapped air pockets between the ice and a specific surface.
An experiment using an array of aluminum square pillars in a controlled environment was used to produce the Cassie state.
The mechanism is believed to involve the presence of water within porous frost tips enabling air pockets to form below the frost.
Removal of ice from surfaces continues to be very challenging, particularly for machinery operating under low-temperature conditions. The presence of ice on the surface of machinery operating at elevated temperatures can increase the possibility of melting, leading to water contaminating a lubricant. This possibility can lead to machinery failure, as water may promote oxidation of the lubricant and facilitate hydrolysis of key components.
In addition, ice present on aircraft wings during flight and on roadways can be dangerous. A previous TLT article
1 discussed a new approach for ice removal known as passive anti-frosting. Researchers determined that application of ice onto a metal surface in the form of stripes will attract moisture present in the environment to preferentially migrate to the ice stripes. The reason for the success of passive anti-frosting is that dry zones surrounding ice form keeping exposed surfaces free from condensation and frost.
Passive anti-frost was found to be effective in a controlled environment where aluminum surfaces were kept at -10 C for up to 24 hours in the presence of a supersaturated water environment. Approximately 90% of the aluminum surfaces containing ice stripes remained frost free.
Current approaches for facilitating the removal of ice from surfaces have focused on the use of nanostructured superhydrophobic surfaces. Jonathan Boreyko, assistant professor of mechanical engineering at Virginia Tech in Blacksburg, Va., says, “About 10 years ago, micro- and nanostructured, superhydrophobic surfaces started to be developed to combat ice buildup. The reason for this approach was the tendency for supercooled water to become suspended in the Cassie state on superhydrophobic surfaces, which will improve the ease of removal through enhancing droplet mobility before freezing. But neither micro- nor nanostructured surfaces are effective under rugged conditions in environments where ice formation is known to occur.”
Boreyko, who led the development of passive anti-frosting, indicates that there may be alternative approaches to generate Cassie states in severe environments without the use of superhydrophobic structures. He says, “Cassie’s Law was discovered about 80 years ago and used to explain how droplets could bead up into spheres on certain textured surfaces. On a smooth surface, droplets can never achieve a contact angle greater than 120 degrees even on a hydrophobic coating such as tetrafluoroethylene. Cassie explained that a structured surface can boost the contact angle to nearly 180 degrees by trapping air pockets underneath the droplet.”
The Cassie state can help to facilitate, as the adhesion strength of ice is much weaker, when air pockets are trapped beneath. Boreyko and his colleagues have now developed a technique for suspending ice on air but without the use of a superhydrophobic surface.
Cassie ice
The researchers envisioned that a Cassie state can be prepared to facilitate ice removal through the placement of an array of aluminum square pillars in a controlled environment where condensation frosting occurs under specific temperature and humidity conditions. Hyunggon Park, graduate research assistant at Virginia Tech, says, “Aluminum square pillars with dimensions of 1.0 millimeter in height and 0.5 millimeter in width are placed on the chilled substrate, and the vapors in the air preferentially condensate on top of the pillars
(Step 1 in Figure 3). As this upper condensate freezes, it evaporates any underlying conditions due to the hygroscopic nature of ice
(Step 2) to result in Cassie frost
(Step 3).
Park continues, “As rain and fog drop- lets impact the surface, this will wick inside of the Cassie frost and get arrested, preventing impalement
(Step 4). As droplets continue to impact the surface
(Step 5), the result is a continuous layer of Cassie frost/ice suspended above air pockets
(Step 6).”
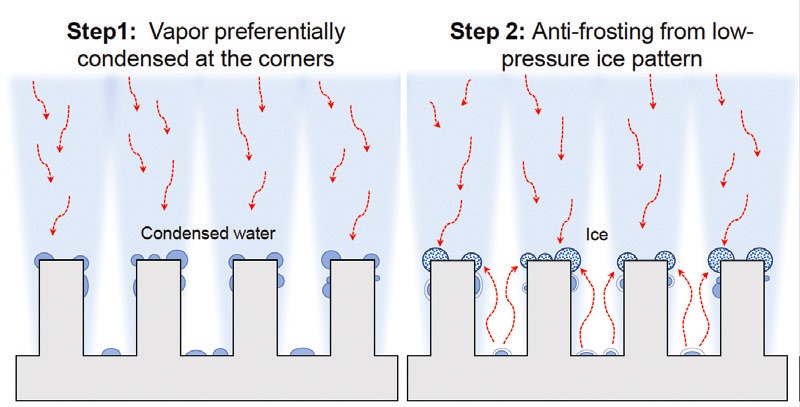
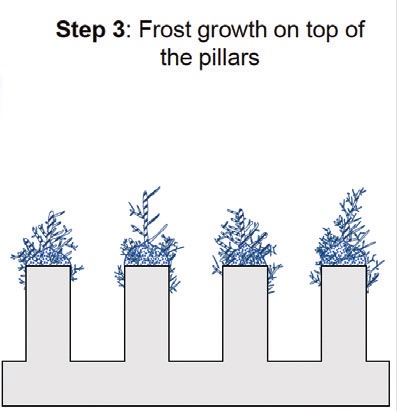
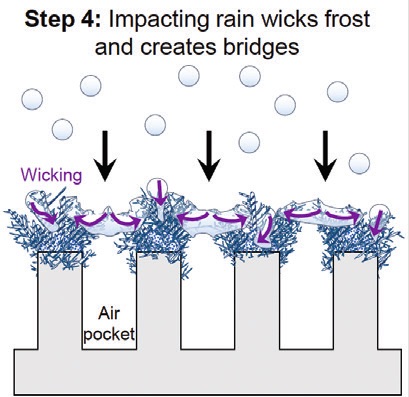
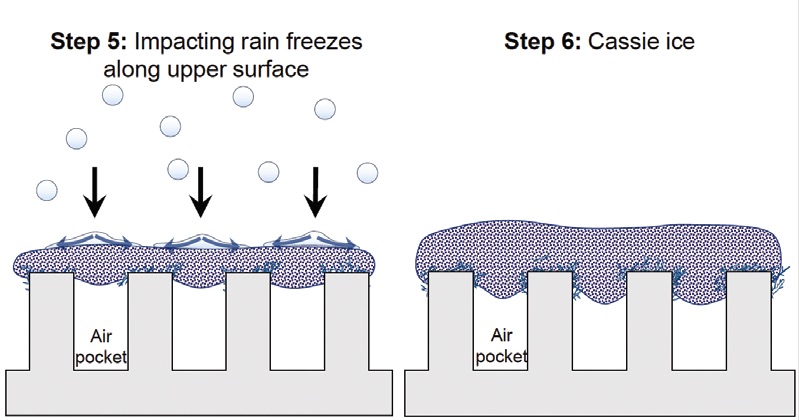 Figure 3. A six-step process is shown for how Cassie ice can form on a surface making removal easier. Figure courtesy of Virginia Tech.
Figure 3. A six-step process is shown for how Cassie ice can form on a surface making removal easier. Figure courtesy of Virginia Tech.
The researchers conducted experiments to attempt to empirically produce Cassie frost/ice under two sets of conditions. The first attempt was in the lab, where the air and impacting water droplets were at room temperature while the pillars were chilled on a cold stage. After frost grew preferentially on the tops of the aluminum pillars, subsequent introduction of water droplets led to their impalement between the pillars (no Cassie ice). This is because the frost tips simply melted when a water droplet impacted them, such that they could not suspend the droplet.
Repeating the same process in a walk-in freezer, where the air and water droplets were additionally chilled, produced the desired Cassie ice. The researchers evaluated three different potential mechanisms and determined that that frost has porosity and acts in the same fashion as a sponge. Boreyko says, “We believe that a wicking mechanism takes place that enables water to become present within the porous frost tips. The result is that air pockets can be formed below the frost leading to the formation of the Cassie state.”
Boreyko envisions that this approach can be used to remove ice in a number of applications including aircraft and machinery. He says, “The geometry of the pillar needs to be taken into consideration if used on an aircraft wing so as not to interfere with the aerodynamics.”
Future work will entail determining how readily the Cassie frost/ice can be removed from the aluminum pillars. Additional information can be found in a recent article
2 or by contacting Boreyko at
boreyko@vt.edu.
REFERENCES
1.
Canter, N. (2018), “Using ice to remove ice,” TLT,
74 (12), pp. 14-15. Available
here.
2.
Park, H. Ahmadi, S. and Boreyko, J. (2021), “Using frost to promote Cassie ice on hydrophilic pillars,”
Physical Review Letters, 127 (4), 044501.