Strategically placing ice stripes along a surface can minimize frost formation in a process known as passive anti-frosting.
Due to ice’s depressed vapor pressure, dry zones form around the ice stripes, keeping the surface free from condensation and frost.
Any surface can potentially be kept frost free through use of passive anti-frosting.
Publication of this article as another winter in the Northern Hemisphere is upon us reminds many of us about the challenges faced in dealing with removing snow and ice from automobiles and roadways. Frost in particular can be more than just a nuisance as its presence can reduce the mechanical integrity and economic efficiency of power grids, wind turbines, aircraft and air conditioning systems. This can place more stress on the lubricants required in all of these applications.
Farzad Ahmadi, graduate student in the department of biomedical engineering and mechanics in the College of Engineering at Virginia Tech in Blacksburg, Va., reports that frost can reduce efficiency of refrigerators and heat exchangers by 75% and increase drag in aircraft by 90%.
Three mechanisms are now available for removing frost from a surface. Ahmadi says, “Application of a high level of heat to melt the ice or, use of mechanical energy to remove ice or as seen more frequently, hygroscopic chemicals such as salts and glycols can inhibit ice nucleation on surrounding surfaces. Two problems with using these chemicals is they are diluted over time as they absorb vapor reducing their effectiveness, and they are not environmentally friendly.”
Ahmadi continues, “Another mechanism that most researchers have been working on is to introduce a superhydrophobic surface that can delay the start of frost formation. The problem with this technique is that frost will eventually propagate on the surface throughout the population of supercooled dew droplets.”
In a previous TLT article, ice growth was determined to be dependent upon the hydrophobicity of a surface (
1). Researchers found that if the contact angle (a measure of hydrophobicity) was greater than 40 degrees, then ice formed in an off-surface growth mode defined as being iceophobic. This type of growth enables air pockets to be generated between the ice and the surface leading to easier removal.
A new technique is needed to more efficiently remove ice, which minimizes the amount of heat and chemicals required and does not utilize superhydrophobic surfaces. Such a technique has now been developed.
Passive anti-frosting
Jonathan Boreyko, assistant professor in the department of biomedical engineering and mechanics, and his colleagues have prepared a new method for ice and frost removal, which in essence involves the use of ice. Ahmadi says, “We have found that by applying ice to a surface in the form of stripes, the moisture available to form frost all over the surface preferentially migrates to the ice stripes. Compared to other hygroscopic chemicals, ice is not getting diluted over time. This environmentally friendly approach is known as passive anti-frosting because no energy needs to be applied to overcome the frost.”
The key to this technique is that dry zones surround ice due to the depressed vapor pressure of ice. Ahmadi says, “Utilizing a surface with the ice stripes, the dry zones overlap keeping the surface free from condensation and frost. The idea is to prevent ice bridging from occurring as frost tries to propagate on a surface in that manner.”
The researchers designed an experiment to prove this concept using uncoated aluminum surfaces. Microgrooves that were 15 microns in width and 25 microns in depth were cut on top of the aluminum surfaces using a laser to form fins. These aluminum surfaces were placed in a humidity chamber where water was then added through the use of a micropipet and the temperature dropped to -20 C to form the ice strips.
The researchers then held the temperature of the aluminum surfaces at -10 C while supersaturating the chamber with water for up to 24 hours. Top-down and side-view microscopes and cameras were used to record the data as shown in Figure 2 for an experiment that lasted for three hours. No ice or frost was observed on the stripes after three hours. The ice seen in the floor and vertical walls of the aluminum surfaces in the side-view images is only present at the terminal end of the fin array.
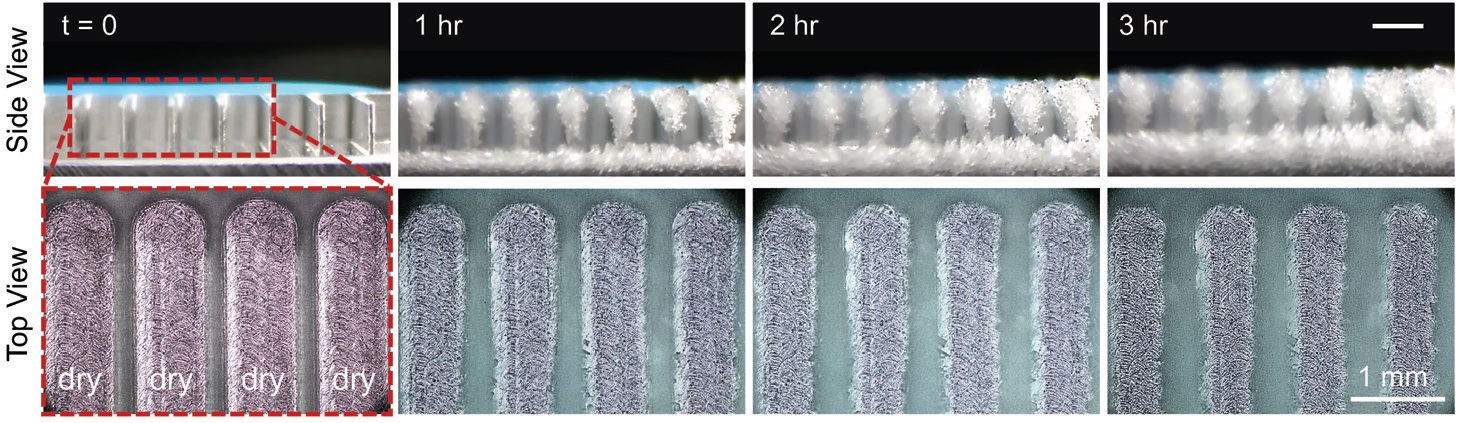 Figure 2. No ice or frost is observed on an aluminum surface kept at -10 C for three hours as shown in the top view on the bottom. The ice and frost observed in the side-view images on the top is only present at the terminal end of the fin array. (Figure courtesy of Virginia Tech.)
Figure 2. No ice or frost is observed on an aluminum surface kept at -10 C for three hours as shown in the top view on the bottom. The ice and frost observed in the side-view images on the top is only present at the terminal end of the fin array. (Figure courtesy of Virginia Tech.)
Ahmadi says, “Even after 24 hours, the ice stripes minimized frost formation so that a stable frost-free area of about 90% of the aluminum surface was identified. We demonstrated the efficacy of the passive anti-frosting technique without using any heat or a special surface coating. We are just using the unique chemistry of ice to prevent condensation from forming.”
Under identical conditions, an untreated smooth aluminum surface without the ice patterns completely frosted over in less than one hour. A superhydrophobic nanostructure was applied to the aluminum surface and evaluated in the same manner. Superhydrophobicity was only effective for a limited period of time before the surface was frosted over; although dew drops were immediately formed on these substrates.
The researchers believe that any surface can be kept frost free through carefully applying a pattern of ice stripes strategically so that the dry zones overlap. They feel that this effect will be seen at any surface temperature below freezing and almost any possible ambient humidity.
Ahmadi says, “We have been approached to develop an ice pattern surface on heat exchanger pumps and airplane wings. One other application that will be examined is to evaluate passive anti-frosting on an automobile windshield for an OEM.
Additional information can be found in a recent article (
2) or by contacting Boreyko at
boreyko@vt.edu.
REFERENCES
1.
Canter, N. (2018), “Relationship between wettability and ice removal,” TLT,
74 (2), pp. 12-13.
2.
Ahmadi, S., Nath, S., Iliff, G., Srijanto, B., Collier, C., Yue, P. and Boreyko, J. (2018), “Passive Antifrosting Surfaces Using Microscopic Ice Patterns,”
Applied Materials & Interfaces,
10 (38), pp. 32874-32884.