A review article has been published that discusses the advantages of using pulsed laser in liquids synthesis.
This technique produces nanoparticles through a process that starts with the generation of a plasma when a pulsed laser beam interacts with a solid immersed in a liquid.
One important application for pulsed laser in liquids synthesis is the production of nanocatalysts that can facilitate sustainable energy technologies.
Catalysts perform important functions in the development and use of lubricants. They vary in size, but, typically, smaller catalysts, particularly those with diameters on the nanoscale, are the most effective because they have higher surface areas and can more efficiently convert specific substrates to desired products.
In a previous TLT article,
1 catalyst size was found to be an important factor in research evaluating the performance of noble metals that convert hydrocarbon combustion by-products from an internal combustion engine into a carbon dioxide and water. The catalysts initially studied were varying combinations of palladium and platinum at five different sizes of nanoparticles ranging from 2.3 to 10.2 nanometers. Further work showed that larger sized nanoparticles in the 10-20 nanometer range exhibit superior performance than smaller nanoparticles because the larger catalysts were able to change shape during the process to become more effective.
With particle size such an important factor, imagine a technique that can control the type and size of nanoparticles produced for use in catalysis. Such a technique was originally developed in the late 1980s and is known as pulsed laser in liquids synthesis. In a recently published review article,2 Astrid Müller, assistant professor of chemical engineering at the University of Rochester in Rochester, N.Y., and her colleagues discussed the advantages of using pulsed laser in liquids synthesis to provide researchers with the opportunity to use this technique in their own work.
Müller says, “Pulsed laser in liquids synthesis involves a pulsed laser beam hitting a solid immersed in a liquid that generates a high-temperature, high-pressure plasma near the surface of the solid. As the plasma, confined within the liquid surrounding it, decays, this leads to the formation of a cavitation bubble as molecules vaporize in the surrounding liquid under the high-temperature and pressure conditions that develop. This bubble is unstable and is prone to periodic expansions and contractions that eventually lead to the generation of shock waves and a violent implosion. The result is rapid cooling that creates the environment for the formation of nanoparticles. Chemical reactions initiated between particles formed from the liquid and particles ablated or knocked loose from the solid create the nanoparticle. Once synthesized, the nanoparticles will be injected into the surrounding liquid and become stable.”
Figure 2 shows an image of the pulsed laser in liquid process with the laser beam (shown in green interacting with a solid in yellow) that leads to the formation of the nanoparticles.
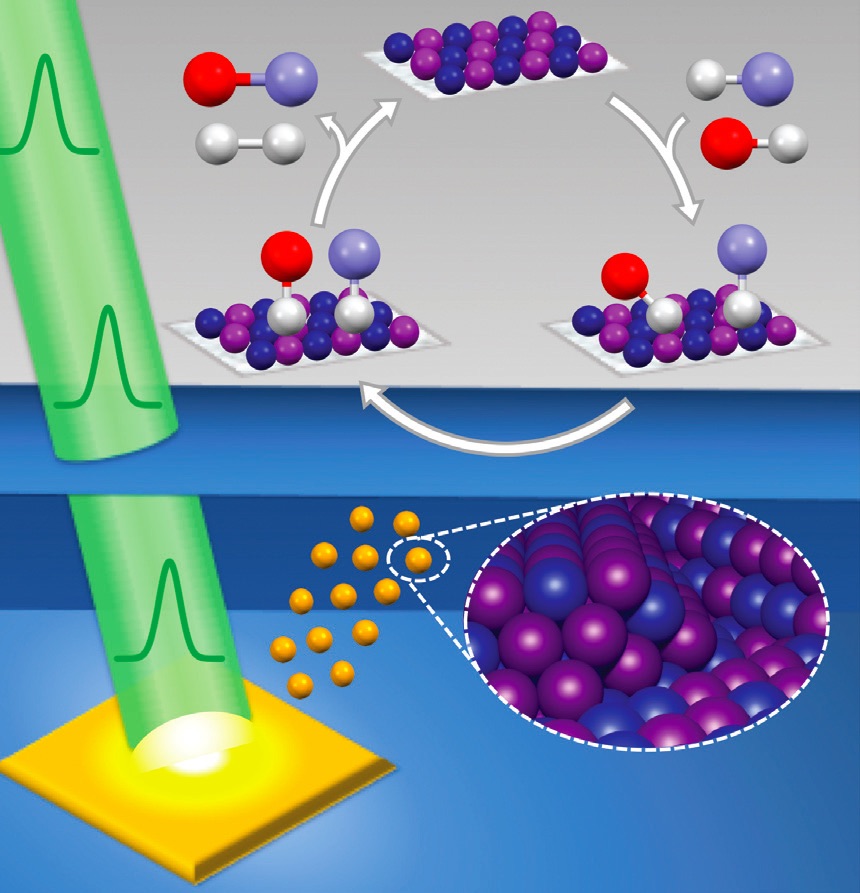 Figure 2. Pulsed laser in liquids starts with a pulsed laser beam (shown in green) striking a yellow solid to produce uniform nanoparticles (in brown). These nanoparticles can be used as catalysts. Figure courtesy of the University of Rochester.
Figure 2. Pulsed laser in liquids starts with a pulsed laser beam (shown in green) striking a yellow solid to produce uniform nanoparticles (in brown). These nanoparticles can be used as catalysts. Figure courtesy of the University of Rochester.
Pulsed laser in liquids synthesis can be used to produce nanoparticles that exhibit consistent properties including size, composition and crystal phase. Müller says, “In contrast to wet chemical synthesis of nanoparticles, no surfactants are required. The nanoparticles formed are consistent in size and tend not to agglomerate once formed.”
Substrate solids that can be used include a variety of metals and derivatives such as metal oxides—in fact, any solid, even colloids, can serve as a target. While water is a common solvent used, other nonaqueous solvents also can be used as long as the conditions are tailored properly to form the nanoparticles.
Müller lists several key advantages for using pulsed laser in liquids. She says, “Development of catalysts can be done more readily using pulsed laser in liquids synthesis than standard wet chemical processing. Typically, 10 catalyst candidates can be produced in one day. Such an effort will take months using conventional techniques. The nanocatalysts produced by pulsed laser in liquids synthesis are more active and more stable than those produced using wet chemical techniques.”
Müller continues, “Metastable catalysts can be produced through the use of pulsed laser in liquids synthesis. The laser can provide enough energy and rapid cooling to synthesize catalysts that would not be accessible using wet chemical methods. Pulsed laser in liquids synthesis also can be used to make mixed metal catalyst of specific sizes. This ability to produce nonequilibrium materials can lead to the preparation of additional, unique high entropy alloys. A final benefit is the ability to use automation in pulsed laser in liquids synthesis, which will provide the opportunity for a more efficient process that can be done remotely, providing cost savings without sacrificing performance.”
Müller feels that her group’s most important contributions in pulsed laser in liquids synthesis is the development of nanocatalysts for use in facilitating sustainable energy technologies. For example, Müller’s group has worked on the development of nonprecious water oxidation catalysts. Water oxidation is the splitting of water into hydrogen and oxygen. She adds, “A second area that we have worked in is carbon dioxide reduction catalysis where the objective is to turn climate-damaging carbon dioxide into useful chemicals and fuels. The pulsed laser in liquids technique is well suited to producing more catalyst candidates in a much shorter period of time. This potentially makes pulsed laser in liquids synthesis an attractive approach as researchers are stepping up their efforts to find ways to cost effectively commercialize sustainable processes.”
Müller indicates that pulsed laser in liquids synthesis could possibly be used to facilitate tribochemistry in specific applications. She says, “The challenge will be to situate a small laser within the application so that it can affect the formation of a nanocatalyst that can interact with the lubricant and the metal surface.”
Müller is optimistic about the future of pulsed laser in liquids synthesis. She says, “Catalysts are the basis for every manufacturing process. This is why we prepared the review to highlight the potential for using pulsed laser in liquids synthesis and to discuss many of the applications where it has been used and can be leveraged in the future.” Besides the review, further information can be obtained by contacting Müller at
astrid.mueller@rochester.edu.
REFERENCES
1. Canter, N. (2020), “Determination of active vehicle exhaust catalyst sites,” TLT,
76 (11), pp. 14-15. Available
here.
2. Forsythe, R., Cox, C., Wilsey, M. and Müller, A. (2021), “Pulsed laser in liquids made nanomaterials for catalysis,”
Chemical Reviews, 121 (13), pp. 7568-76