A lack of understanding about how noble metals catalyze hydrocarbon combustion byproducts has hindered attempts to improve the performance of catalytic converters.
Active sites in palladium/platinum nanocrystal catalysts were identified through the use of experiments that modeled oxidation in a diesel engine.
Large catalyst nanoparticles between 10 and 20 nanometers are superior catalysts because of their ability to change shape during the oxidation reaction, leading to the formation of more active catalyst sites.
Emissions produced by automobiles continue to be a concern, and global regulations have been established and continue to be updated to reduce their impact on the environment. At the core of controlling exhaust emissions is the three-way catalytic converter based on noble metals that include palladium, platinum and rhodium.
As noted in a previous TLT article,
1 the standard catalytic converter was designed in the 1940s. This article describes the development of a new catalytic converter technology that is based on microstructured ceramic substrates. By using this structure, the researchers were able to improve the effectiveness and durability of the catalyst. Most importantly, a lower quantity of noble metals was required for the catalytic converter to be as effective as those used commercially.
Matteo Cargnello, assistant professor of chemical engineering at Stanford University in Palo Alto, Calif., says, “Palladium and platinum have remained as an important catalyst in catalytic converters because of their excellent stability and high efficiency in treating automotive emissions. The automotive industry requires catalysts that will operate effectively over the lifetime of a vehicle that can operate for 150,000 miles or more.”
One of the problems faced by researchers is the lack of understanding about how noble metals actually catalyze the conversion of hydrocarbon combustion byproducts to carbon dioxide and water at the atomic level. Cargnello says, “Currently used catalysts exhibit poorly defined metal sizes and compositions, which makes identifying the active sites where oxidation of hydrocarbons occurs efficiently difficult to find.”
Knowing the most active sites can allow for an increase in their density, thus, optimizing and reducing the use of expensive noble metals in current converters.
One hydrocarbon that is generated during the combustion reaction is propene. Cargnello says, “Propene is a major species emitted from the internal combustion engine and is representative of the components that are neutralized by the catalytic converter. This simple alkene also is more stable due to the presence of a double bond, which is difficult to break during the oxidation reaction and that covers the metal surfaces, leading to decreased converter efficiency.”
A new approach has now been developed to better understand the mechanism for how propene is converted to carbon dioxide and water and to identify the active sites in the noble metal catalyst that facilitate this process.
Reductionist approach
Cargnello and his colleagues at Stanford and the Department of Energy’s SLAC National Accelerator Laboratory have now identified active sites on palladium/platinum nanocrystal catalysts that can accelerate the reaction of propene with oxygen. He says, “Trying to study catalysts and figure out how they work is a very complicated task that involves a large number of experiments. We used a reductionist approach that accelerated the process by combining experiments with theoretical studies and the use of analytical techniques to characterize active sites. Instead of working with the actual emissions stream, we decided to simplify the study by working with a model system containing propene and catalysts used at known concentrations and particle sizes supported on common, thermally stable oxide materials such as alumina.”
The researchers focused on modeling oxidation in a diesel engine, which meant the focus was on varying the atomic ratios of platinum and palladium and using lean combustion experimental conditions (oxygen-to-propene ratio of 20). Initial work was conducted with three catalyst atomic ratios (palladium to platinum ratios of 3/1, 1/1 and 1/3) in the absence and presence of steam (10% by volume). The characterization tools and collaborations at SLAC National Laboratory were crucial to obtain the correct particle sizes and compositions required for the study.
Cargnello says, “Steam (water) plays an important role in the combustion reaction. We initially found that steam adversely impacts the ability of palladium to function by binding the catalyst and not enabling it to react with oxygen. In contrast, steam does not affect the performance of platinum.”
The researchers then decided to more closely determine how the size of catalyst nanoparticles affect performance. Five different sizes of nanoparticles ranging from 2.3 to 10.2 nanometers supported on alumina were evaluated. Cargnello says, “Density functional theory analysis was conducted to assist us with determining what shapes the particles display under reaction conditions, a very difficult task to accomplish for experiments.”
In this next stage of experiments, the atomic ratio of platinum to palladium was set at 1/1. Cargnello says, “We decided to use equal atomic ratios of the two elements to give the catalyst balance. Palladium will more readily activate oxygen while platinum is superior at activating propene.”
Further experimentation showed that large catalyst nanoparticles between 10 and 20 nanometers displayed superior catalysis than smaller nanoparticles. Cargnello says, “This result seems counterintuitive because smaller nanoparticles typically have larger surface areas for reactions to occur for a given amount of metal. But water appeared to block these catalyst sites. In contrast, larger particle sizes change shape during the oxidation reaction, resulting in the formation of more active catalyst sites that counterbalance the lower surface area.”
Images in Figure 2 clearly show how a nanoparticle changes in shape. Figure 2 shows a simulated palladium, platinum nanoparticle on the left before the reaction and in the middle after the reaction. A transmission electron microscopy image of a 10.2 nanometer particle is shown on the right.
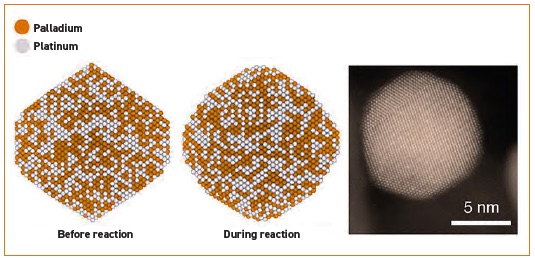 Figure 2. During the combustion oxidation reaction, nanoparticles containing palladium and platinum (see left image) change to a rounder shape (see middle image) exposing more active catalyst sites. A transmission electron microscope image of the rounder catalyst nanoparticle is shown on the right. Figure courtesy of Stanford University.
Figure 2. During the combustion oxidation reaction, nanoparticles containing palladium and platinum (see left image) change to a rounder shape (see middle image) exposing more active catalyst sites. A transmission electron microscope image of the rounder catalyst nanoparticle is shown on the right. Figure courtesy of Stanford University.
The active sites also are undercoordinated, which means that there are open sites where reactants can engage with the metal catalyst. Cargnello explains, “Palladium and platinum are transition metals that form face centered cubic structures with a coordination number of 12. A fully coordinated metal has no ability to act as a catalyst. Open spaces on surface atoms that are representative of undercoordinated sites display the potential for a high level of catalytic activity. In the oxidation of propene, step edges in the nanoparticles with a coordination number of 7-7 contain extra sites available for oxidation of propene.”
Finding where reactions occur on catalyst surfaces is crucial to developing more efficient systems.
Future work will involve examination of other hydrocarbon substrates such as propane, which is similar in chemical structure to propene but does not have a double bond and to replace platinum and palladium in larger nanoparticles with metals that are less expensive and more readily available. Cargnello says, “Our objective is to develop vehicle exhaust catalysts that are more effective, contain more readily available metals and can be used on the broad classes of hydrocarbons that are produced during combustion.”
Additional information can be found in a recent article
2 or by contacting Cargnello at
mcargnello@stanford.edu.
REFERENCES
1. Canter, N. (2014), “New catalytic converter technology,” TLT,
70 (6), pp. 14-15.
2. Yang, A., Choksi, T., Streibel, V., Aljama, H., Wrasman, C., Roling, L., Goodman, E., Thomas, D., Bare, S., Carrera, R., Schäfer, A., Li, Y., Pederson, F. and Cargnello, M. (2020), “Revealing the structure of a catalytic combustion active-site ensemble combining uniform nanocrystal catalysts and theory insights,”
Proceeding of the National Academy of Sciences, 117 (26), pp. 14721-14729.