HIGHLIGHTS
•
A review article discussed the current methods for recovering lithium from spent batteries as an approach to minimize safety and environmental concerns.
•
The three methods available for recycling lithium-ion batteries are pyrometallurgy, hydrometallurgy and direct recovery. Each of these methods has limitations but can lead to a 17%-61% reduction in greenhouse gas emissions.
•
More work needs to be done to improve the efficiency of recycling, including designing more suitable battery chemistries and structures.
The growing use of lithium-ion batteries in applications such as electric vehicles is leading to challenges about how to source lithium cost-effectively and then what should be done at the end of the battery’s operating life. As stated in a recently published review article,
1 lithium production increased from 16.4 metric tons in 2021 to 84,000 metric tons in 2022 and is estimated to reach 914,000 metric tons in 2030. Demand for lithium is estimated to increase by 18 times in 2030 and by 60 times in 2050.
The lead author of the review article, Asad Ali, graduate student in the school of engineering at Edith Cowan University in Perth, Western Australia, says, “Lithium-ion batteries effectively operate in electric vehicles until their capacity drops to 80% over time. This means that only 20% of the battery has been consumed. Recycling appears to be a suitable alternative as compared to landfilling lithium-ion batteries due to safety and environmental concerns and due to the potential to regenerate.”
Ali indicates that lithium-ion batteries require greater than 99.5% pure battery grade lithium salts.
The review article provides a comprehensive analysis of the methods available for recovering lithium and potential recycling options from the technological, economic and environmental standpoints.
To make the argument for recycling, Ali and his colleagues first discussed the difficulties in isolating lithium from mining (it is present in rocks at concentrations ranging from 20 to 70 ppm), and in brine (present at a concentration of approximately 1,000 ppm). Ali says, “Among the prominent hard rocks that contain lithium is the mineral spodumene which is the primary lithium bearing silicate mineral—a series of processing steps that include mechanical separation, forth flotation and then magnetic separation.”
Based on a review of known references, the researchers conclude that mining generates 20.4 tons of carbon dioxide equivalents of carbon dioxide per ton of spodumene.
Isolation of lithium from brine is conducted by the use of solar evaporation ponds. This technique utilizes sunlight to isolate lithium derivatives such as lithium hydroxide at higher concentrations up to 6,000 ppm, but impurities can cause complications according to Ali. This process generates a lower rate of emissions (2.7-3.1 tons of carbon dioxide per ton of lithium carbonate) than mining but consumes a higher quantity of water.
Ali and his colleagues conclude that using either mining or evaporation causes significant land disruption, soil contamination and exhibits large ecological, water and carbon footprints.
Recycling methods
Pyrometallurgy, hydrometallurgy and direct recovery are the three methods that have been identified to recycle lithium-ion batteries. While each of these methods has its advantages and disadvantages Ali believes there is room for making recycling more efficient.
The main objective of pyrometallurgy is to recover valuable metals from the cathode using high temperature. Precious metals such as cobalt and nickel are isolated from the spent battery based on their specific melting and boiling temperatures. Ali says, “The main concern with using pyrometallurgy is that this technique does not provide a strategy for also recycling lithium from a spent battery. Another negative issue is that the high temperatures required lead to the release of toxic gases such as hydrofluoric acid which originate from the battery’s electrolyte.”
Hydrometallurgy is a much more favorable recycling method from a sustainability standpoint according to Ali. He says, “After pre-treatment and physical separation of the spent lithium-ion battery, an acidic leaching step is conducted to systematically isolate key metals including lithium, cobalt, nickel, manganese and phosphorus. Once leaching is accomplished, separation and purification steps are needed to isolate the specific metals in the lithium-ion battery.”
Currently inorganic acids such as hydrochloric and sulfuric are used in hydrometallurgy. Ali adds, “These acids are difficult to work with because they are corrosive to users and can produce harmful by-products such as chlorine and sulfur dioxide. We believe that moving to organic acids is a better approach due to reduced concern about corrosion, lower energy requirements and limited environmental concerns. Candidates that are under evaluation include citric acid, oxalic acid and lactic acid.”
Direct recovery literally means retrieving the lithium-ion battery’s cathode in its original form without doing any operations to change its structure. There are various methods that can be used but all of them are at the earliest stage (lab scale).
A past TLT article
2 discussed a new approach for direct recovery. Researchers recognized its limitations and decided to utilize froth flotation to isolate two common cathodic materials: a mixture of lithium, cobalt, nickel and manganese oxides (NMC III) and lithium manganese oxide (LMO). An 80% recovery rate was achieved in experiments conducted with a 1:1 mixture of the two cathode materials.
Figure 4 provides a summary of the three methods used to recycle lithium-ion batteries and the products that are obtained from the cathodes.
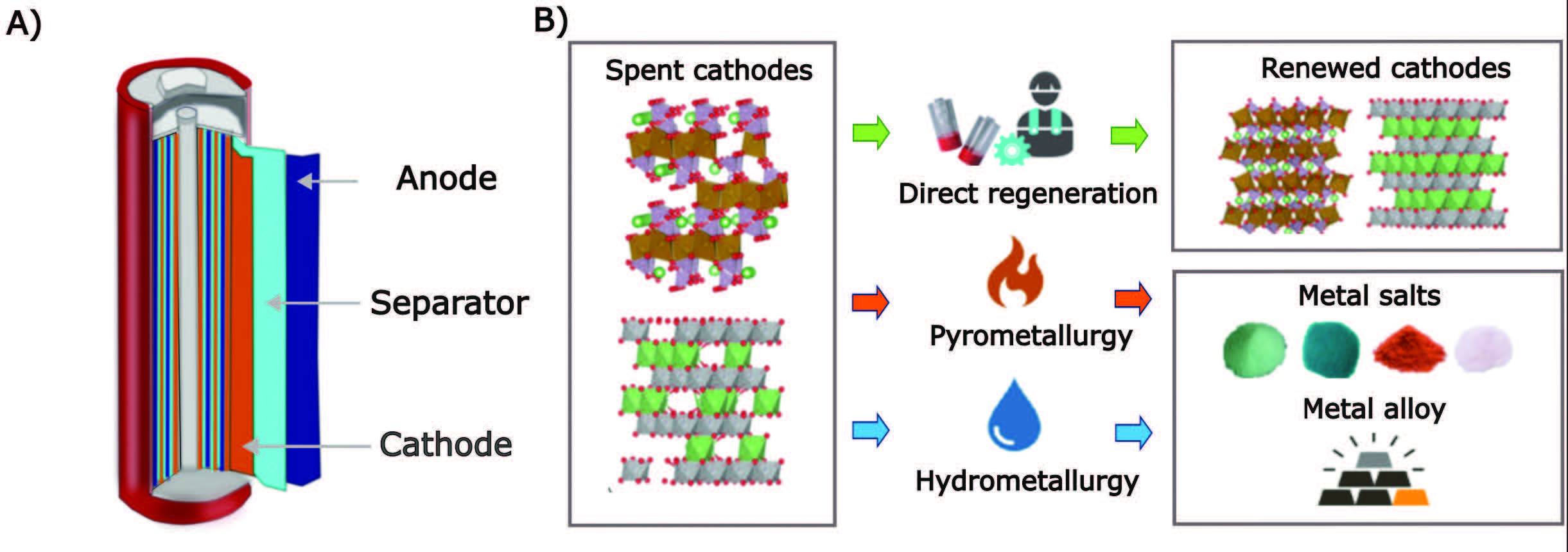 Figure 4. To recycle the cathode of a lithium-ion battery, three methods are available that can produce metal salts and alloys which will then be available for future use in a new battery. Figure courtesy of Edith Cowan University.
Figure 4. To recycle the cathode of a lithium-ion battery, three methods are available that can produce metal salts and alloys which will then be available for future use in a new battery. Figure courtesy of Edith Cowan University.
A fourth recycling method, biological metallurgy extraction, is under development. Ali says, “This method involves using microorganisms (such as bacteria and fungus) to extract metals from waste lithium-ion batteries. Biological metallurgy extraction is cost effective and very efficient, but developing a commercial process is still a work in progress.”
The researchers conclude that recycling lithium-ion batteries can lead to a 17%-61% reduction in greenhouse gas emissions depending upon the method used. Capital costs and water consumption are much lower.
Ali says, “More work needs to be done to improve the efficiency of recycling. This involves designing battery chemistries and structures that are easier to recycle.” Additional information can be found in the review article
1 or by contacting Dr. Muhammad Rizwan Azhar, lecturer at Edith Cowan University at
m.azhar@ecu.edu.au.
REFERENCES
1.
Ali, A., Afrin, S., Asif, A., Arafat, Y. and Azhar, M. (2025), “A comprehensive review on the recovery of lithium from lithium-ion batteries and spodumene,”
Journal of Environmental Management, 391, 126512.
2.
Canter, N. (2022), “Battery cathode recycling: Froth flotation,” TLT,
78 (2), pp. 12-13. Available at
www.stle.org/files/TLTArchives/2022/02_February/Tech_Beat_I.aspx.