HIGHLIGHTS
•
Megalibraries are emerging as high-throughput tools for material synthesis and screening.
•
This technique positions over 150 million well-defined nanoparticles on a 2 centimeter by 2 centimeter chip which can be evaluated for a specific performance parameter in a relatively short period of time.
•
A case study found that use of a megalibrary led to the identification of a catalyst that exhibits superior properties in the oxygen evolution reaction than the current industry accepted catalyst, iridium dioxide.
Inorganic nanoparticles are under evaluation for use in a number of applications. This column has discussed how nanoparticle tribology is being exploited to build new types of structural materials.
However, it can be time consuming to identify the right nanoparticle for a specific application because, at least theoretically, the options are nearly infinite—spanning a range of sizes and compositions encompassing all the possible combinations of the elements of the periodic table.
Chad A. Mirkin, George B. Rathmann professor of chemistry (Weinberg College of Arts and Sciences) and director of the International Institute for Nanotechnology (IIN) at Northwestern University in Evanston, Ill., says, “The chemical, electrical, mechanical and structural properties of nanoparticles are significantly different from the bulk versions of materials of the same compositions.”
Mirkin and his colleagues discovered a new strategy for rapidly identifying specific nanoparticles of interest. He says, “We introduced the concept of a megalibrary, millions of positionally encoded nanoparticles on a 2 centimeter by 2 centimeter chip. The particles systematically vary in size, composition and structure and, therefore, in their chemical or physical properties.”
The researchers can position over 150 million well-defined nanoparticles on a specific surface in the course of an afternoon. Mirkin says, “Megalibraries are made by preparing arrays of 250,000 nanoscale tips (pyramids) then using them to write nanoreactor patterns on chips through a combination of polymer pen lithography (PPL) and scanning probe block copolymer lithography (SPBCL). These nanoreactors can be converted into nanoparticles, and this method ultimately allows us to make more new types of materials in a single experiment than humans have collectively synthesized to date.”
An illustration of a nanoparticle megalibrary is shown in Figure 1.
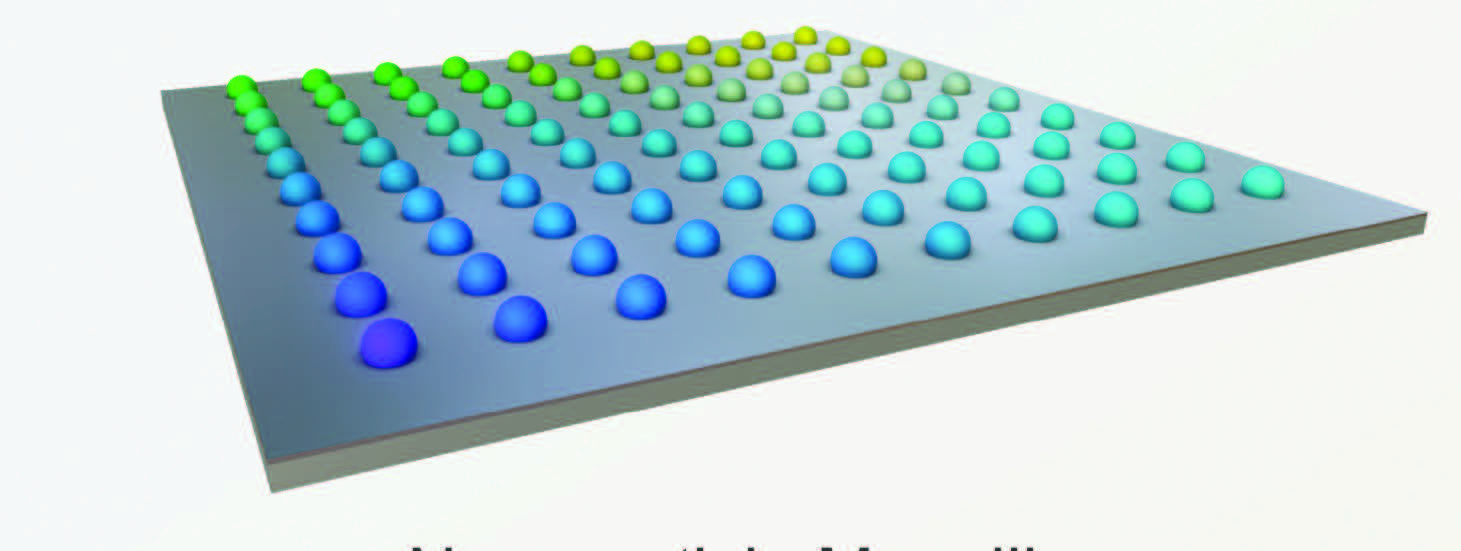 Figure 1. This schematic of a megalibrary demonstrates the positioning of a large number of nanoparticles on a specific surface. Figure courtesy of Northwestern University.
Figure 1. This schematic of a megalibrary demonstrates the positioning of a large number of nanoparticles on a specific surface. Figure courtesy of Northwestern University.
The use of a megalibrary as a high-throughput tool for materials synthesis and screening stands in contrast to other techniques such as inkjet printing. Mirkin says, “Inkjet printing produces structures that are too big and insufficient to provide an efficient approach to resolve a specific issue. Typically, features are limited to the 100-25,000 features per library. A good analogy is that the number of features inkjet printing will need to produce to meet what is done in a single megalibrary would fill an area the size of a football field.”
Mirkin reports that the megalibrary concept has been used primarily to identify catalysts for electrochemical-induced water splitting, carbon nanotube generation and record blue and white-light photoluminescence so far. He adds, “In collaboration with Toyota Research Institute and MattIQ, we have also used massive data sets gleaned from megalibraries to train machine learning models.”
He notes that megalibraries can be applied to find new types of catalysts for any chemical reaction.
A new study
1 has just been conducted to illustrate how megalibraries can be used to identify novel catalysts for the oxygen evolution reaction, or OER.
Alternative to iridium catalysts
Renewable hydrogen production by splitting water is hindered by the efficiency of the OER. The key process hindering hydrogen formation is the OER. A previous TLT article
2 discussed an alternative approach, where a renewable carbon source, such as biomass, is utilized in a reaction with water to produce hydrogen and carbon dioxide.
However, proton-exchange membrane water electrolyzers, where the main catalyst for OER is iridium dioxide, are most often used for water splitting. Mirkin says, “The challenge in working with iridium dioxide is its limited availability globally and high cost. Efforts are underway to find alternative catalysts such as those composed of ruthenium dioxide. While this candidate can exhibit higher catalytic activity than iridium dioxide, stability is a major problem.”
Mirkin and his colleagues used the megalibrary concept to find a catalyst that is much more active than iridium dioxide but is also durable and widely available. The researchers constructed a four-element megalibrary with approximately 156 million individual nanoparticles, totaling 250,000 unique chemical compositions.
The four elements employed in this study were ruthenium (Ru), cobalt (Co), manganese (Mn) and chromium (Cr). Mirkin says, “These elements were selected because they are more readily available than iridium, lower in cost and have been evaluated as promising in previous oxygen evolution catalyst studies.”
The nanoparticles were prepared by spray coating a quaternary ink gradient consisting of metal salt precursors derived from the four elements and an organic block copolymer on a tip array. The tip array was used to pattern nanoreactors on a carbon substrate. Metal oxide nanoparticles were generated by thermally annealing the nanoreactors at 625°C for 6 hours under a hydrogen atmosphere, and then subjecting them to air oxidation at 400°C.
The researchers divided the megalibrary into four quadrants, each of which were predominantly based on one of the elements. The average size of the resulting nanoparticles ranged from 16 to 20 nanometers.
An initial rapid screening of all 156 million nanoparticles was conducted using an automated programmable scanning electrochemical method that delineated nanoparticle catalytic activity for the OER. One candidate, Ru
52Co
33Mn
9Cr
6 oxide, was identified as displaying the highest level of activity.
A schematic that shows this catalyst splitting water is provided in Figure 2.
 Figure 2. A specific catalyst based on ruthenium, cobalt, manganese and chromium was identified in a megalibrary as exhibiting the highest level of water splitting catalytic activity. Figure courtesy of Northwestern University.
Figure 2. A specific catalyst based on ruthenium, cobalt, manganese and chromium was identified in a megalibrary as exhibiting the highest level of water splitting catalytic activity. Figure courtesy of Northwestern University.
Scaled-up forms of this catalyst underwent quantitative measurements of activity, and the researchers found that it displayed better catalytic activity compared to iridium dioxide. Importantly, the identified catalyst also was found to be stable and active when incorporated into a proton-exchange membrane water electrolyzer.
Mirkin says, “This case study demonstrates the enormous potential in using the megalibrary approach for materials discovery. We identified a previously unknown catalyst based on ruthenium, cobalt, manganese and chromium in just one afternoon of testing. This study explored a vast compositional and structural space, and it did so in a way not possible with serial methods at a fraction of the cost.”
Mirkin says that the megalibrary concept can be applied to identify materials useful in solving challenging issues encountered by tribologists and lubrication engineers. Additional information on the megalibrary concept can be obtained by contacting Mirkin directly at
chadnano@northwestern.edu.
REFERENCES
1.
Huang, J., Wang, Z., Liang, J., Li, X., Pietryga, J., Ye, Z., Smith, P., Kulaksizoglu, A., McCormick C., Kim, J., Peng, B., Liu, Z., Xie, K., Torrisi, S., Montoya, J., Wu, G., Sargent, E. and Mirkin, C. (2025), “Accelerating the pace of oxygen evolution reaction catalyst discovery through megalibraries,” Journal of the American Chemical Society,
147 (34), pp. 30956-30966.
2.
Canter, N. (2024), “Hydrogen production from renewable sources,” TLT,
80 (12), pp. 18-19. Available at
www.stle.org/files/TLTArchives/2024/12_December/Tech_Beat_III.aspx.