Preparation of solid-state batteries has been hindered by limitations on charge transport due to the potential of various interfaces that may impede their movement.
Introduction of ionically conductive polymer additives into a ceramic matrix material, using a method known as cold sintering, produced a solid-state electrolyte that exhibits high ionic conductivities.
The presence of the ionically conductive polymer dispersed uniformly through the ceramic matrix reduces interfacial resistance.
Concerns about working with lithium-ion batteries that contain liquid electrolytes has been leading researchers to work to develop alternatives based on solid-state electrolytes. The expectation is that solid-state electrolytes are more stable, and the threat of a failure leading potentially to a short circuit and a fire is minimized. Solid-state batteries also have the potential to exhibit greater energy density, meaning they can extend the operating range of a battery electric vehicle between charges.
Development of solid-state batteries has been hindered by limitations on charge transport due to the potential of various interfaces that may impede their movement. This includes cathode-electrolyte, electrolyte-boundaries, anode-electrolyte and additive-electrolyte interfaces that also lead to the degradation of battery performance.
Ceramics appear to be a good material for use in the preparation of solid-state electrolytes. Hongtao Sun, assistant professor in the Harold and Inge Marcus Department of Industrial and Manufacturing Engineering, and the Materials Research Institute at Penn State University in State College, Pa., comments on their benefits and limitations. He says, “Ceramics are mechanically robust. But they have limitations in not being able to effectively bridge interfaces, leading to increased interface resistance.”
Sun adds, “The current state of the art process for preparation of ceramic solid-state electrolytes is to utilize sintering to compress the ceramic materials into a uniform, highly dense form. The process involves the use of high temperatures (between 800-1,200°C) and long processing times (several hours). The sintering temperature required is approximately 80% of the melting points of the ceramic materials.”
The researchers found that adding ionically conductive polymer additives into a ceramic matrix material led to the formation of a polymer-in-ceramic composite that can produce a densified solid-state electrolyte with reduced interface resistance. The problem faced in making a ceramic matrix composite electrolyte is that the high temperatures and long processing conditions are not cost effective nor favorable to integrate components that are thermochemically reactive under current sintering conditions. Sun says, “A new ceramic composite manufacturing approach needs to be developed to more efficiently prepare solid-state electrolytes that are more electrically conductive especially across the interfaces.”
To achieve this goal, Sun and his team employed a method known as cold sintering, a process that involves heating powdered materials, adding a small amount of liquid solvent, and applying pressure to produce a denser structure. Drawing inspiration from geological solution-precipitation creep, this approach allows high-temperature ceramics to be densified alongside polymer additives at much lower processing temperatures (e.g., 150°C) than those required for conventional sintering. The introduction of a minimal amount of solvent creates hydrothermal micro-environments that promote mass transport and enhance the densification process.
The ceramic used in this study is based on lithium, aluminum, titanium and phosphate and is known as NASICON-phase, Li
1.3Al
0.3Ti
1.7(PO
4)
3 (LATP), which is commonly used in the preparation of solid-state electrolytes. The polymer is a highly conductive poly (ionic liquid) gel. Both are prepared in a glove box
(see Figure 4) to minimize air and water contamination. The cold sintering set up is shown in Figure 5.
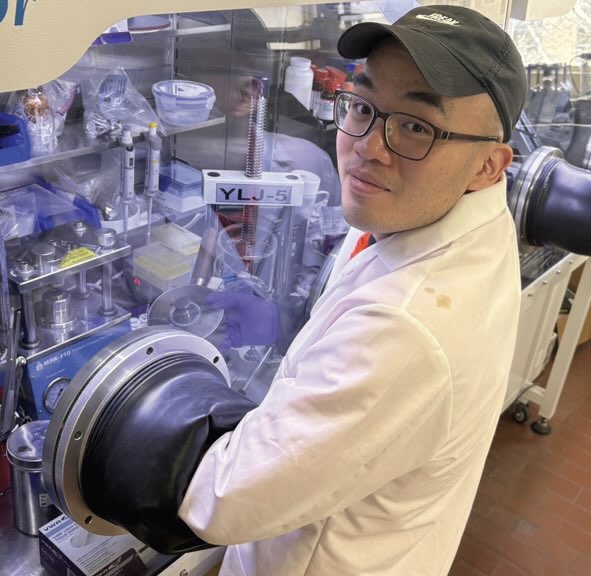
Figure 4. The solid-state electrolyte displaying promising performance characteristics was prepared with highly conductive poly (ionic liquid) gel and a ceramic in an inert atmosphere (glove box). Figure courtesy of Penn State University.
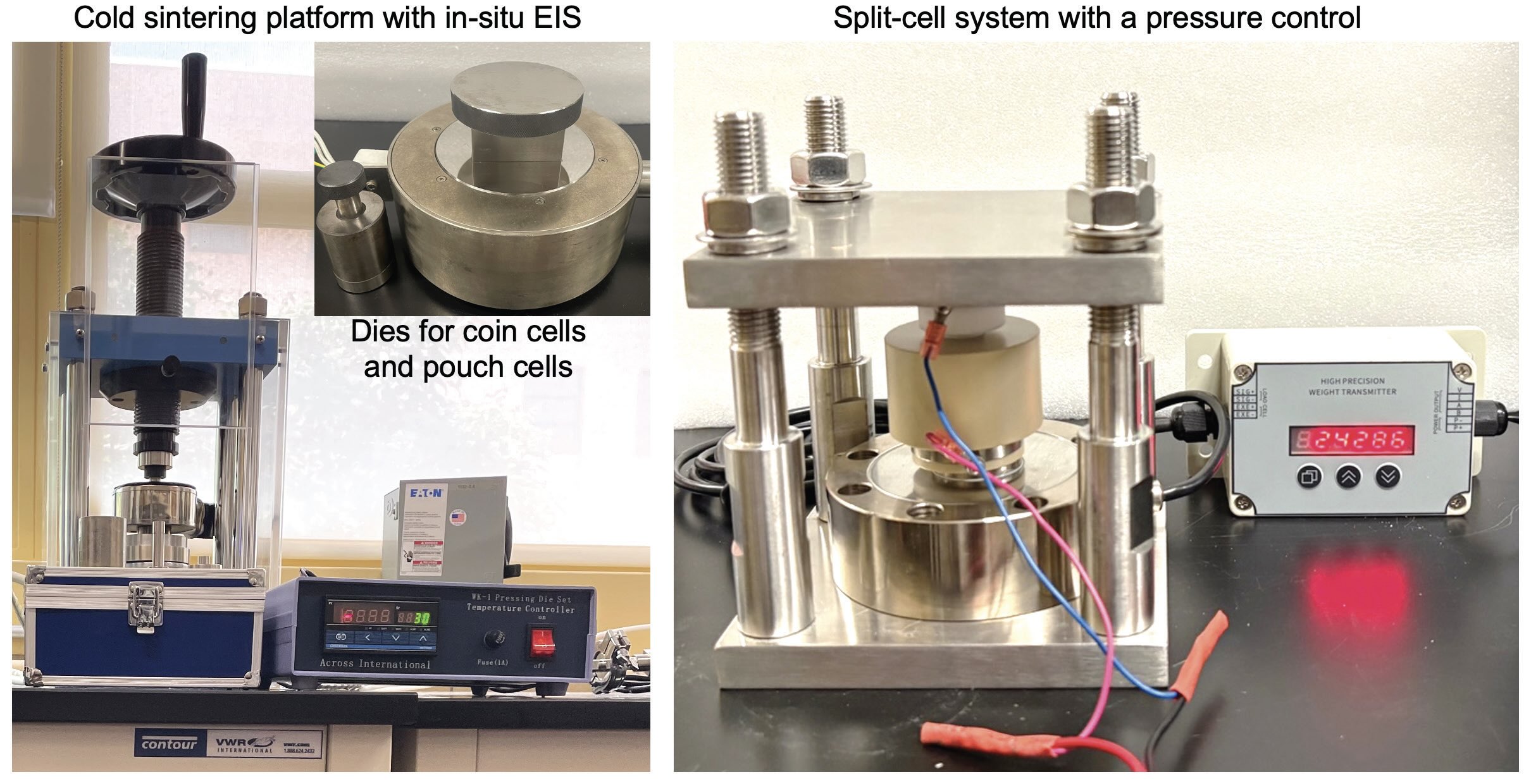
Figure 5. Cold sintering was used to prepare the solid-state electrolyte. Figure courtesy of Penn State University.
Sun says, “The presence of the poly (ionic liquid) gel in the ceramic composite reduces interfacial resistance because the polymer phase is present on the boundaries of the LATP particles. Cold sintering ensures that the polymer gel is deployed uniformly through the ceramic matrix.”
The result is a solid-state electrolyte that exhibits high ionic conductivities of 4.2 X 10
-4 Siemens per centimeter in a coin cell and 5.15 x 10
-4 Siemens per centimeter in a split cell under 20 megapascals of pressure at room temperature. Sun says, “We determined that the voltage range, a measure of energy density, for the solid-state battery is 0-5 volts, which is larger than the voltage ranges seen for lithium-ion batteries containing liquid electrolytes (0-4 volts).”
In this particular study, dimethyl formamide (DMF) was used as a transient sintering aid. Sun says, “DMF’s presence during cold sintering helps to facilitate mass transfer enabling the integration of the ceramic composite. Once the process is completed, most of the DMF used evaporates meaning there is very little left in the ceramic composite. Due to health and safety concerns, we will be evaluating other solvents including water to improve the cold sintering process.”
This approach for preparing a solid-state electrolyte brings the probability of commercializing solid-state batteries for use in applications such as electric vehicles closer to reality. Sun says, “Our next objective is to scale-up the cold sintering process using small-sized dies from the lab to a batch process suitable for commercialization. A large-sized custom die has been constructed to be used in preparing punch battery cells. Eventually, we are working to develop a continuous process.”
A second objective is to better understand if the solid-state electrolyte can be reused. Sun says, “Recycling could emerge as a simple cost-effective method to introduce this solid-state electrolyte by retaining the key components for future use.”
Additional information on this work can be found in a recent article
1 or by contacting Sun at
hongtao.sun@psu.edu.
REFERENCE
1.
Nie, B., Wang, T., Lee, S., Zhang, J. and Sun, H. (2025), “Probing cold sintering-regulated interfaces and integration of polymer-in-ceramic solid-state electrolytes,”
Materials Today Energy, 49, 101829.