Current methods for extracting lithium, including extraction from hard rock, and filtering brine, are currently challenging.
Keggin polycation produces an aluminum-pillared material suitable as a membrane for extracting lithium.
Doping this membrane with sodium cations facilitated the separation of monovalent cations (lithium and sodium) from the multivalent cation, magnesium.
Increasing the ratio of sodium to lithium cations enabled the two elements to be separated by the aluminum-pillared vermiculite membrane.
The growing demand for lithium metal and lithium-ion batteries is leading to a growing need to develop an economically viable approach for extracting lithium so that it can be readily converted into a form for use in these applications. Current methods for isolating lithium are difficult to achieve, energy intensive and time consuming.
The leading strategies are to mine and process spodumene ore and to extract lithium from water, which is challenging because the element is present at low concentrations. In addition, lithium is in environments where other elements such as sodium and magnesium are present at higher concentrations. Separating these specific cations from each other can be extremely difficult.
Seth Darling, chief science and technology officer for the Advanced Energy Technologies directorate at Argonne National Laboratory in Lemont, Ill., says, “There are currently two approaches for obtaining lithium. The element can be extracted from hard rock through mining, which occurs primarily in Australia. But this process is difficult to scale further due to high energy usage and environmental concerns. Lithium can also be extracted by pumping brine from underground into massive ponds in the ‘Lithium Triangle’ located in Argentina, Bolivia and Chile. These ponds are so massive that they can be seen from space. Despite being located in a desert, evaporation of water from these ponds to process the lithium can take from nine to 18 months. Moreover, there are many untapped brines with lithium salts around the world, but these often have even lower concentrations of lithium.”
Filtering the brine mixture containing lithium through an appropriate membrane has emerged as a potentially more efficient extraction option. Darling says, “Common membranes used to desalinate seawater remove all the salts rather than separating lithium from the other cations present in the water. Ideally, a membrane would selectively transport lithium and reject the other ions (or vice versa). But this is nearly impossible using current commercially available membranes because the elements have similar charge and size.”
Darling and his colleagues decided to explore a family of naturally occurring two-dimensional materials known as phyllosilicates (clays) as the source materials for membranes. He says, “These materials are worth evaluating because they are available at large scale, are low in cost and have the potential to be engineered to fabricate precise membranes. But they are also very hydrophilic which means they tend to fall apart when exposed to water.”
The researchers took one of the most abundant phyllosilicates, vermiculite, and transformed it into a membrane material that is both stable in water and can isolate lithium from magnesium and sodium.
Aluminum-pillared vermiculite membranes
A water stable vermiculite-based membrane was prepared by treating thin layers of the phyllosilicate with an aluminum cation hydrolysis product known as the Al
13 Keggin polycation. The vermiculite was prepared by exfoliation leading to the formation of a uniform dispersion of two-dimensional flakes with an average lateral size of approximately 1 micron and a monolayer thickness of 1.23 nanometers. Strong electrostatic interactions between the vermiculite and the aluminum hydrolysis product yielded a crosslinked vermiculite structure. The strong electrostatic attraction between the negative vermiculite flakes and the positive Keggin ion produced a stable stack with the latter sandwiched between layers of the former. These Keggin ions were then transformed into robust pillars between the layers by heating in an oven
(see Figure 3).
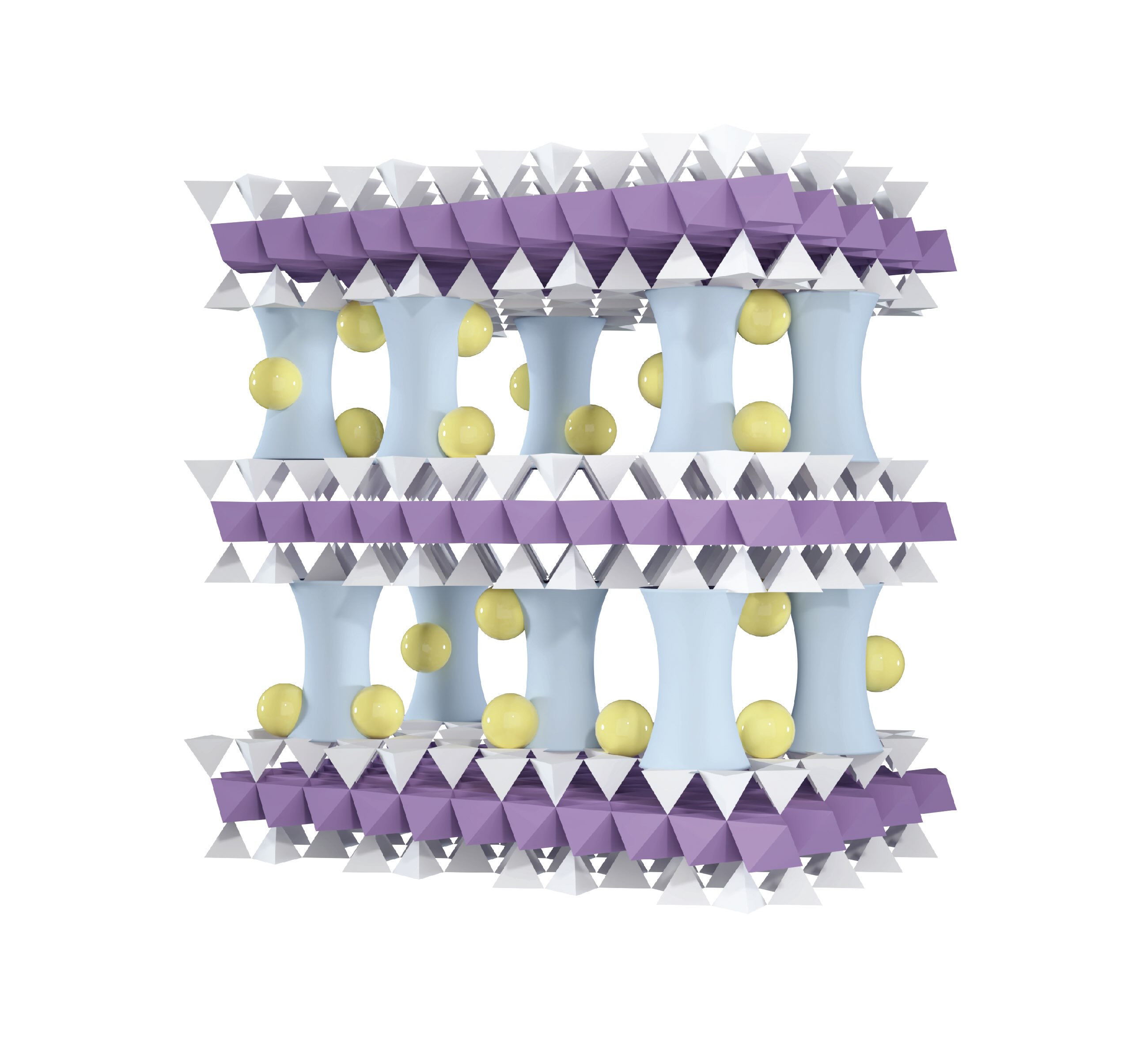
Figure 3. Addition of sodium atoms (shown as yellow balls) into the aluminum-pillared vermiculite membrane is critical to enable lithium atoms to be extracted from magnesium and sodium atoms. Figure courtesy of Argonne National Laboratory.
The laminar structure of the vermiculite membrane is maintained, and the pillared material was found to remain stable after soaking in a 0.1 molar solution of sodium chloride for 30 days. Further testing demonstrated the stability of the aluminum-pillared vermiculite membrane in sodium chloride solutions up to a concentration of 5 molar and at pH values ranging from 3 to 12.
Darling says, “The aluminum-pillared vermiculite membrane still needed to be fine-tuned to facilitate the separation of lithium cations from magnesium and sodium cations. Our next step was to determine how to separate monovalent (+1) lithium cations from multivalent (+2) magnesium cations. To undertake this challenge, the Donnan exclusion mechanism was utilized, which involves separating ions through electrostatic repulsion of the higher valence species.”
This objective was achieved by doping the vermiculite membrane with sodium cations (see yellow balls representing sodium ions in Figure 3) during the aluminum pillaring process. Experimentation showed that the membrane’s permeability for lithium did not change but the permeability of magnesium ions declined by 14 times. Darling says, “The doping effect of sodium enhanced the degree of positive surface charge leading to a more effective rejection of the ion with the greater positive charge, magnesium.”
Separating monovalent lithium and sodium ions is not doable from an electrostatic standpoint. The researchers then turned to figuring out how to separate them based on their size difference. Darling says, “The size of the two cations in water is based on their hydrated structures. Hydrated lithium is actually subtly larger in size than hydrated sodium. We adjusted the size of the transport channel in the aluminum-pillared vermiculite membrane by adding more sodium cations to the membrane after the pillaring process.”
Increasing the ratio of sodium to lithium cations from 1 to 10 leads to an increase in the permeable membrane selectivity of sodium to lithium from 8.6 to 21. This result probably occurs because the increase in dopant cations reduces the size of membrane pores to more effectively exclude the larger hydrated lithium cations while allowing the smaller sodium ions to move through.
Darling indicates that future work will focus on scaling up this process so it can be used commercially. He says, “Current membrane fabrication has been done using vacuum filtration in the lab, which is not feasible on an industrial scale. We have recently succeeded in using a slot die coating to prepare crosslinked vermiculite membranes. Manufacturing the membrane through the use of a roll-to-roll process is now the next step.”
Pressure and electrodialysis are also under evaluation to speed up the actual lithium extraction process through the aluminum-pillared vermiculite membrane. Additional information can be found in a recent article
1 or by contacting Darling at
darling@anl.gov.
REFERENCE
1.
Liu, Y., Wang, Y., Sengupta, B., Kazi, O., Martinson, A., Elam, J. and Darling, S. (2025), “Pillared laminar vermiculite membrane with tunable monovalent and multivalent ion selectivity,”
Advanced Materials, 37 (14), 2417994.