In theory, an inert atmosphere will prevent lubricant oxidation, solving multiple problems. Will other problems arise?
Test methods that assume the presence of an oxidizing atmosphere might have to be modified or redesigned for lubricants under an oxygen-poor atmosphere.
Academic groups, lubricant suppliers and OEMs must collaborate to develop a solid business case for lubricant inerting.
If lubricant oxidation is such a big problem, why not just take away the oxygen? This idea has been around for more than 50 years, but, like many good ideas, putting it into practice back then was more difficult than anticipated.
Commercial supersonic airliners made their appearance in the late 1960s, although they had been used for military and research purposes before then. NASA researchers, concerned about the high operating temperatures for supersonic aircraft, investigated the possibility of running their lubricated systems under a nitrogen atmosphere. It was a good idea on paper. In practice, the nitrogen leaked around various seals to such an extent that aircraft would have had to carry tanks of compressed nitrogen, adding to the weight, expense and potential hazard of such systems. A 1969 report dismissed the idea as impractical,
1 and it was set aside.
That’s not to say that no one has been doing research on lubricants and solid surfaces in the absence of oxygen over the past decades. Several research groups have been studying solid and liquid lubrication and tribofilm formation under vacuum and inert gases
2-4 (see Figure 1).
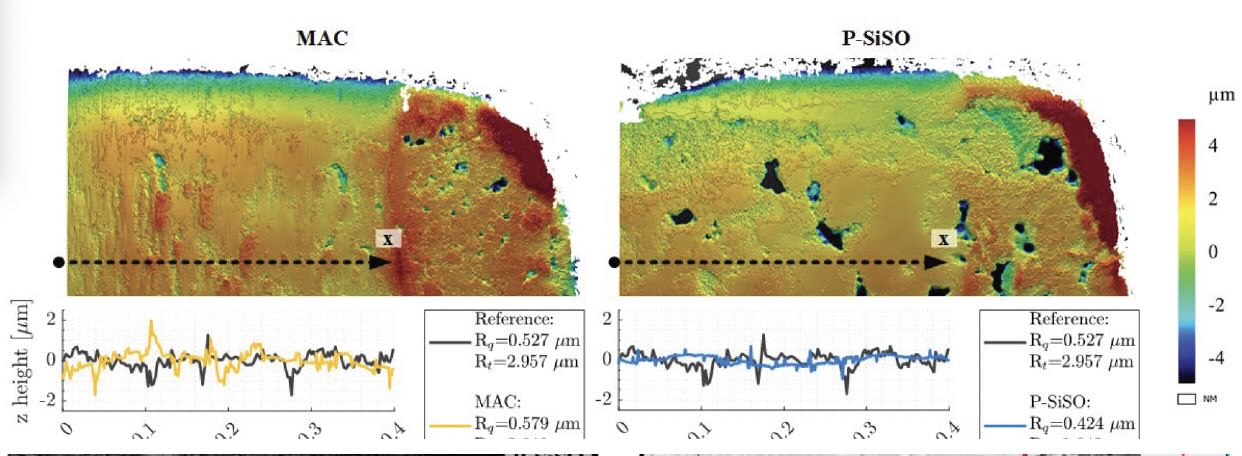 Figure 1. Surface roughness profiles were compared for gear tooth surfaces that had been run under a nitrogen atmosphere under lubricant-starved conditions. Multiply alkylated cyclopentane (MAC), a lubricant used in space applications, was used without (left) and with (right) a tribofilm-forming ionic liquid additive (P-SiSO). The MAC-only surface shows evidence of scuffing, while the P-SiSO surface shows smooth contact patches and retains the porous structure of the unworn sintered material. Figure courtesy of Reference 4.
Figure 1. Surface roughness profiles were compared for gear tooth surfaces that had been run under a nitrogen atmosphere under lubricant-starved conditions. Multiply alkylated cyclopentane (MAC), a lubricant used in space applications, was used without (left) and with (right) a tribofilm-forming ionic liquid additive (P-SiSO). The MAC-only surface shows evidence of scuffing, while the P-SiSO surface shows smooth contact patches and retains the porous structure of the unworn sintered material. Figure courtesy of Reference 4.
Meanwhile, other researchers and engineers have developed small, relatively inexpensive nitrogen concentrators. These adsorption- and membrane-based concentrators make it possible to extract almost pure nitrogen from the ambient air, eliminating the need for tanks of compressed or liquid nitrogen for many laboratory instruments, food packaging applications and other uses. However, only in the past few years have industrial researchers and engineers begun thinking in terms of using membrane-type nitrogen concentrators to provide inert atmospheres for use in large mechanical systems.
For example, in March 2025, a major aircraft manufacturer patented nitrogen-jacketed hydrogen supply lines for aircraft that run on hydrogen fuel cells.5 In their patented system, a concentrator supplies nitrogen to outer pipes that surround inner pipes carrying hydrogen. The nitrogen in the outer pipes provides a barrier that prevents hydrogen leaks from reaching the ambient air, as well as keeping atmospheric oxygen from contaminating the hydrogen supplies. In the event of a hydrogen leak, sensors monitoring the nitrogen stream for hydrogen contamination sound an alert.
Nitrogen concentrators: Necessary but not sufficient
If nitrogen concentrators are small enough and safe enough to carry aboard an aircraft, could they be installed in land vehicles or other mechanical systems? Lubricant oxidation currently presents a significant challenge to formulators, especially as original equipment manufacturers (OEMs) increasingly think in terms of fill-for-life systems. But if there is no contact with oxygen, it stands to reason that there is no oxidation. Currently most gearboxes contain ambient air from the initial assembly, pressure regulation breathers and oxygen permeation through the seals. However, today’s gearboxes are usually sealed for greater efficiency, leak and contamination prevention and longer service lifespans, so in theory, maintaining a nitrogen-rich environment inside should be possible.
At present, nitrogen concentrators are relatively large, says STLE Life Member Hugh Spikes, Emeritus Professor and senior research investigator in the Tribology Group at Imperial College London. This isn’t primarily because of any technical requirement, he says. Rather, the main demand today is in large industrial food packaging operations that use nitrogen to keep food fresh in sealed packages. These concentrators operate on a similar principle to the small personal-use oxygen concentrators that saw an upsurge during the COVID-19 pandemic. Medium-sized nitrogen concentrators, about the size of a tower-type PC, are already on the market, says Spikes’ research colleague STLE member Janet Wong, reader at Imperial College London. These units provide nitrogen to laboratory instruments and industrial soldering operations. There’s no reason that nitrogen concentrators couldn’t be made even smaller if there was a market for them, Spikes says.
Tribology is not an issue for inert gases used as supply line jacketing, coolants for data storage centers or dry atmospheres in laboratory equipment. Nitrogen has been used extensively in these applications, and the development of nitrogen concentrators has provided great benefits in terms of cost and availability. Avoiding the need for compressed gas cylinders or cryogenic storage containers also provides safety benefits.
However, running lubricants in direct contact with a nitrogen atmosphere presents numerous questions in areas that have had little to no previous research. If nitrogen eliminates oxygen-driven degradation, does it introduce other reactions with a lubricant? What kinds of tribofilms form under nitrogen? How does a lubricant age under nitrogen? How does an inerted system that runs continuously differ from one that runs intermittently? And the question that most interests OEMs and their customers: How does running lubricants under an inert atmosphere affect the performance and service life of real-life mechanical systems?
Wong notes that providing a nitrogen supply is no longer the main challenge to running lubricants under a nitrogen atmosphere. Today, the challenge is to determine how various lubricants perform in the absence of oxygen. She adds that characterizing this performance in the lab is a vital step before trying to introduce this novel idea to vehicle manufacturers. “So, the difficulty wasn’t about the nitrogen supply,” she says. “It’s to make sure that our lubricant actually can work well in the intended environment, and also to generate confidence for the industry to take up this technology.”
Toward that end, academic researchers have been contacting and collaborating with lubricant suppliers and OEMs to find out their priorities and concerns.
Lubricant companies: Adding value to their products
“Stronger formulations are being made and environments that make molecules last longer,” says Rihard Pasaribu, team leader for lubricant product application specialists at Shell Lubricant Solutions in The Netherlands. As a part of this effort, he is collaborating with the Imperial College group to investigate the effects of running lubricated machinery under a nitrogen atmosphere. His team of engineers works with large customers to help them get the most out of their lubricants, and he is looking at lubricant inerting from the standpoint of price, performance and ease of adoption. Will lubricant customers be able to use the same products under nitrogen that they did under air? How will lubricant inerting affect lubricant performance, operation costs and drain intervals?
Some industrial applications like compressors and turbines already use inert atmospheres or vacuum to help prevent varnish formation. Over-pressuring a system with an inert gas, Pasaribu explains, keeps air out of the system. Applying a vacuum also keeps air out, but a vacuum system needs good seals, and a significant amount of energy is required to maintain the vacuum, he adds. “The fundamental idea is to eliminate oxygen dissolved in the oil, thus eliminate the oxidation.”
However, Pasaribu continues, some currently used additives require oxygen to function properly. The question is “how much oxygen?” he says. Lubricant manufacturers want to avoid having to reformulate their existing products to make them work under nitrogen, he adds, so the challenge is to find the minimum amount of oxygen that a lubricant needs to function. If a company’s existing product portfolio can continue to work well with less oxygen than is in ambient air, it simplifies the company’s supply chains and marketing efforts, while extending lubricant lifetimes and possibly enhancing performance.
Lubricant performance is the most important factor in a customer’s decision whether to adopt lubricant inerting, Pasaribu says. Because the focus is on extending lubricant life and enabling systems to function at higher temperatures using existing products, the main advantage is cost savings from extending drain intervals, reducing downtime and, for some applications, reducing the amount of cooling needed to keep the lubricant functioning well. An additional advantage of these cost-saving measures is that all these factors also reduce the environmental impact of the lubricant.
At this stage of the research, Pasaribu says, his group is focusing on customers who use large volumes of lubricant for critical operations. These customers potentially have the most to gain from adopting inert atmosphere technology, once pilot-scale tests have demonstrated its feasibility and provided quantitative information on the best operating conditions and lubricant products to use. How much energy is required to run the nitrogen concentrators? What environmental impact will this have? What effect will the change have on a customer’s operations? Researchers are moving beyond the basic concepts toward increasing the kinds of molecules that they need to investigate. “I don’t think it’s very early-stage research, but it’s now in the early part of moving toward solutions,” he says.
One area where lubricant inerting could have a beneficial environmental impact is by enabling more widespread use of biobased oils with enhanced biodegradability and lower toxicity profiles, which may meet criteria for environmentally acceptable lubricants. Some of these oils and lubricants, Pasaribu says, cannot match the high-temperature performance of their mineral oil or synthetic hydrocarbon counterparts, at least under air. An inert atmosphere might make it possible for some biobased oils to continue to perform well at higher temperatures. What’s more, a dry nitrogen atmosphere could also keep these oils from hydrolyzing, opening up new applications for existing lubricants.
OEMs: Seeking significant benefits in real applications
Once enough data has been collected on how various lubricant formulations perform under oxygen-poor atmospheres at temperatures typical of real-world mechanical systems, the next step is to evaluate the lubricants in test rigs. If the results are promising, testing can move on to pilot-scale evaluations, and eventually, field tests.
Transmissions and drivetrains for large electric vehicles (EVs)—including trucks, buses and coaches—represent one area where lubricant inerting might provide economic and environmental benefits, says STLE member Jonny Hansen, tech lead for EV tribology for the TRATON GROUP and assistant professor at Luleå University of Technology in Sweden. He notes that although the applied research in this area is still in the early stages, it could move ahead very quickly because of the potential for extending lubricant life and improving performance. His group is interested in potential applications for heavy electric transmissions, but they are still investigating the specific ways that lubricant inerting could provide the most benefit.
Heavy, loaded commercial vehicles face lubricant challenges similar to those for smaller passenger vehicles, but the high cost of the larger vehicles creates an expectation of a much longer service lifetime, says Hansen’s colleague Peter Björklund, technical manager at TRATON GROUP. Commercial vehicles spend most of their time on the road producing income for their owners, so reliable performance and minimal downtime are critical factors. “If you look at the lubricant,” he says, “then you expect good performance, good efficiency and a long drain interval.”
Hardware life is another factor, Björklund adds. A trend toward fill-for-life lubricants can reduce the overall volume of lubricant used over time, but this requires a lubricant that can continue to perform well for extended periods. Preventing rust and corrosion, including copper corrosion in EVs, is a part of extending hardware life, says Hansen, and this is one area where lubricant inerting might prove its practicality.
The commercial vehicle sector is becoming more electrified, especially in Europe, Björklund says, so it will be interesting to see whether lubricant inerting can benefit all types of commercial vehicles, or if it is especially useful in one specific vehicle type or application. In some very heavy operations, high operating temperatures are a concern, so the thermal stability of the lubricant is especially important. A reduced need for cooling could allow some applications to operate at a higher power density, which might improve efficiency.
Getting the right amount of lubricant conductivity is especially important for EVs. EV lubricants must insulate hardware components against damage from stray electric currents, but they must also be slightly conductive to prevent a buildup of static electricity, which could cause arcing. Academic research is in progress on the effects of lubricants in an electrified, oxygen-free or oxygen-poor environment. This includes investigations on the carbon-based tribofilms that form under a nitrogen atmosphere but not under air.
2,3 One such study indicated that changing from ambient air to nitrogen under electrified conditions reduced wear in test pairs by a factor of 8 to 10.
2 At present, though, tribo-electrochemical mechanisms in these environments and their potential effects in lubricated mechanical systems remain insufficiently understood and require further investigation, Hansen says.
Longer drain intervals and reduced lubricant degradation reduce the amount of lubricant required over a vehicle’s life, which contributes to sustainable operations. Another aspect of sustainability involves using environmentally friendly lubricant components. Lubricant inerting could make it possible to use these oils in more applications and under higher operating temperatures. Although EVs have hot spots that require cooling systems, “temperature doesn’t seem to be an issue now,” says Björklund, but “that could quickly change depending on design choices.”
Indeed, while elevated temperatures generally accelerate hydrolysis, temperature is not the only driver. Inerting can facilitate the transition to ester-based oils even at normal operating temperatures by directly targeting the root causes of hydrolysis—namely, moisture and oxidative byproducts, Hansen says.
What happens in an (almost) oxygen-free environment?
Lubricants that function well under an inert atmosphere could contribute to sustainability in several ways. “We may need to tweak our lubricants to meet the new operating environment,” Wong explains. You might be able to dispense with antioxidants and corrosion inhibitors or use less of them. A longer-lasting lubricant wouldn’t have to be replaced as often, and it could enable the mechanisms it lubricates to last longer. “A small change can potentially lead to a large impact,” she adds.
Ideally, a lubricant would not degrade in an oxygen-free environment, but this assumes that lubricant degradation is caused mainly by oxidation, Wong says. However, she adds, other degradation mechanisms might occur in nitrogen, and other types of additives might be required to counteract this.
If oxidation isn’t a problem, you might be able to run a motor or other lubricated system at a higher temperature, which would affect what kind of a cooling system you need, or whether you need one at all. There is some potential for simplifying these systems and for using less energy. Some systems might run more efficiently at higher temperatures, Wong says, but thermal degradation could become more important than oxidative degradation.
In addition, some common lubricant additives (notably, some ashless phosphate-containing antiwear additives) work better under low oxygen levels, some do not work as well, and others appear to be unaffected.
6 Spikes notes that his group talks about low oxygen levels rather than the absence of oxygen because, “the reality is that we’ll never have no oxygen.” Ambient air contains about 78% nitrogen, 21% oxygen and various amounts of water vapor. In general, nitrogen concentrators deliver between 98% and 99.5% nitrogen. However, this is sufficient to deliver the needed benefits, he adds.
Nitrogen is less soluble in typical lubricants than oxygen is. Thus, nitrogen should not contribute to lubricant viscosity changes or foaming or affect the hydrodynamic or elastohydrodynamic lubrication properties.
6 Little research has been done on these questions, but plans are being made to address them. The main issue, Spikes says, is whether a lubricant forms an effective tribofilm under an inert atmosphere. Some work better, some not so well, he says. Also, it’s not clear how inerted lubricants perform under boundary conditions. He and his colleagues are working to understand the effects of an inert atmosphere on lubricants more fully at the bench scale and pilot plant levels before they move on to field tests.
Spikes notes that at 1% oxygen, lubricants can last 20 times as long as they do under air. How do you test lubricant aging at that scale? Can you make a lubricant that lasts for 20 years without losing its properties, or will the additives be used up? “We think that most of the additive is lost by oxidation,” he says. “So, the answer is, we don’t know, but that’s another thing that needs to be checked.” He adds that additive replenishment is already a common practice in various applications. When people think about lubricant degradation, Wong adds, they often focus on the properties of the base fluid. However, a nitrogen environment could also prolong additive life.
The Imperial College group is working to assemble a consortium to work on these questions. They are hoping that the European Union (EU) takes an interest in lubricant inerting, which would make it easier to use bio-derived lubricants like plant oils, which are susceptible to oxidation and hydrolysis. If an atmosphere containing little to no oxygen could reduce the need for toxic amine antioxidants and ZDDP, Spikes says, it would benefit the environment.
Many lubricated systems today are fill-for-life, but lubricants for applications like compressors, hydraulic systems or wind turbines that involve severe contact or harsh environmental conditions must be replaced periodically. Industrial machinery commonly runs continuously, and the hardware service life can span decades. Having a lubricant that lasts significantly longer in these systems could provide significant savings in time and expense, as well as providing environmental and sustainability benefits. In addition, Spikes says, “if you can maintain a slight positive pressure in your gearbox, in your industrial site, you shouldn’t get contamination. Often, what kills an industrial machine is dust, grit or dirt. Having a flow of nitrogen through the headspace will probably minimize that.” Nitrogen also keeps a system dry, which can keep ester-based lubricants from hydrolyzing.
Testing the concept
Long-lived lubricants not only pose challenges for running tests in a reasonable amount of time, but they also might degrade in unexpected ways. Does making one part of a lubricant formulation work longer cause problems with other parts of the formulation to become prominent?
From the standpoint of adapting laboratory equipment, testing lubricants under nitrogen is easier than under hydrogen, ammonia or other flammable or corrosive gases. The Imperial College group tested samples using a high-frequency reciprocating tribometer enclosed in an airtight plexiglass box equipped with gas ports and an oxygen sensor, a modification that is commonly available for many test instruments
6 (see Figure 2).
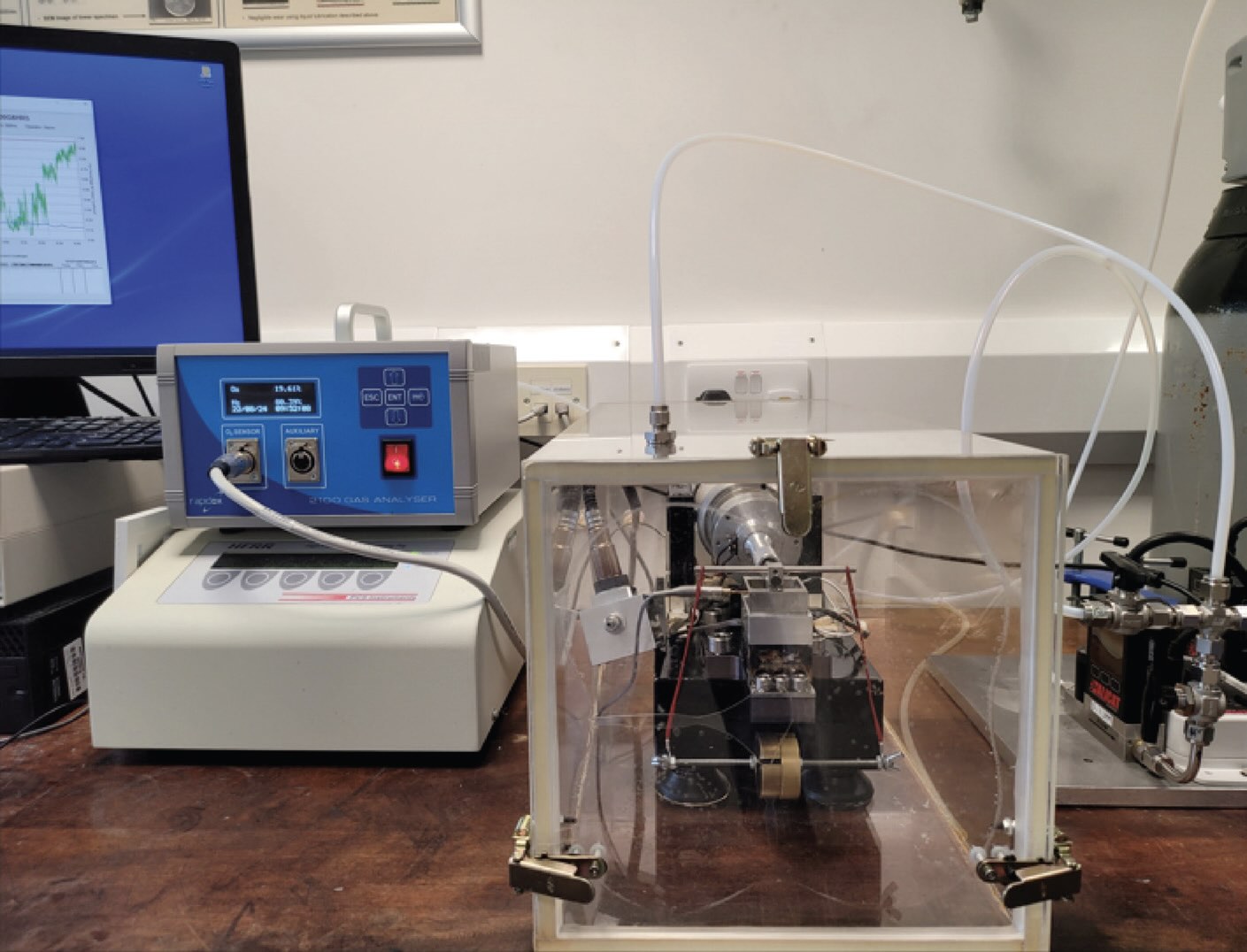 Figure 2. High-frequency reciprocating tribometer in an atmosphere-controlled chamber. Figure courtesy of Reference 6, CC BY license.
Figure 2. High-frequency reciprocating tribometer in an atmosphere-controlled chamber. Figure courtesy of Reference 6, CC BY license.
Adapting the test methods was more challenging because of the very small amounts of oxygen in the samples. Most standard tribology test methods are run under ambient atmosphere. Spikes explains that if oxygen is not present, then the classical form of oxidation (peroxide formation, which then forms acids, ketones, etc.) does not occur. “And we are trying to see what sort of degradation you might get. You might get more unsaturated compounds, but we know it’s very slow, because we’ve done that work already. We’re interested in what you do actually get, because contacts are very severe, and even without oxygen, you still break bonds and form free radicals. It’s just that they don’t react with oxygen to feed the peroxide cycle,” he adds.
In addition to Fourier transform infrared (FTIR) spectroscopy and measurements of total acid number (TAN) and viscosity, the Imperial College group is also exploring the use of ultraviolet (UV)-visible spectroscopy, which is especially good for investigating systems of organic molecules with conjugated bonds. They have tested fully formulated gas turbine oil, hydraulic fluid and automatic transmission fluid, as well as high-oleic sunflower and isostearic acid base oils
(see Figure 3). Their tests so far using multiple methods have shown a small amount of very slow oxidation under an atmosphere of about 2% oxygen (lower oxygen levels are possible, but testing at these levels is more difficult).
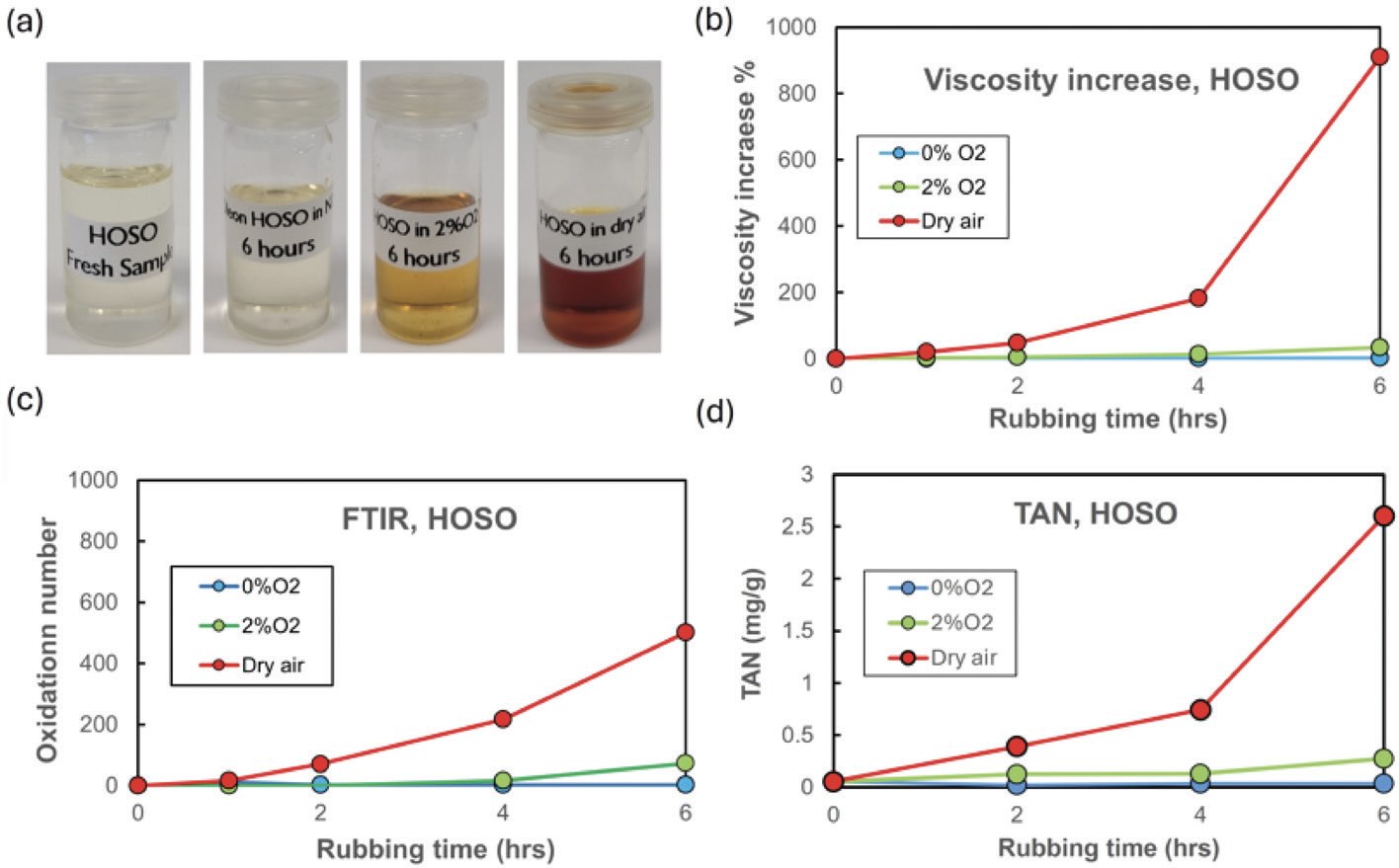 Figure 3. Degradation results for high-oleic sunflower oil (HOSO) base oil tested at 250°C. FTIR is Fourier transform infrared spectroscopy, TAN is total acid number. Figure courtesy of Reference 6, CC BY license.
Figure 3. Degradation results for high-oleic sunflower oil (HOSO) base oil tested at 250°C. FTIR is Fourier transform infrared spectroscopy, TAN is total acid number. Figure courtesy of Reference 6, CC BY license.
The group hopes to begin laboratory testing soon using a full-scale gearbox and a compressor to observe how inerted lubricants behave in a realistic mechanical environment. The next step would be to collaborate with industrial partners who have test rigs that simulate various real-world mechanical systems, Wong says. They are currently working with an industrial partner to set up a hydraulic pilot study, but typical tests like accelerated aging present a challenge. Not only do inerted lubricants last much longer, but the standard test methods for lubricant aging involve bubbling air or oxygen into the system. “How do you test?” Spikes asks, referring to aging tests for systems with little to no oxygen. “You can’t use [standard tests] in the way they are set up.”
Wong notes that it is well established that lubricants last longer under an inert atmosphere. “The question is, does it improve five times or 100 times?” The group will need to run quantitative tests in order to present a convincing case to potential industrial partners. In addition, they will need to document what levels of nitrogen purity provide optimal performance, how often to change the membranes in the concentrators, how these parameters change at various operating temperatures and other practical considerations.
A work in progress
Looking ahead, would inerted lubricants be practical for use in EVs? Unlike internal combustion engines (ICEs), which require oxygen-driven combustion to drive the pistons and which release soot particles into a lubricant, electric motors run in a clean, sealed environment. The copper motor windings are especially sensitive to the peroxides and acids that form in an oxygenated environment. Under a nitrogen atmosphere, it might be possible to reduce or discontinue the use of sulfur-containing additives, which would help to prevent corrosion still further. Other recent research indicates that a nitrogen atmosphere helps to prevent wear in environments with a high electric potential, the type typically seen with EVs.
2
Some research has been done on the tribofilms that base oils form under a nitrogen atmosphere. These films have been difficult to characterize, but the consensus is that they are carbon-based.
3,7 Work is still in progress on characterizing the effects of various solid surfaces and operating conditions, as well as how films formed by a neat base oil differ from those formed by a fully formulated lubricant. Preliminary results indicate that these films are not especially lubricious, and they are not by themselves sufficient to provide adequate protection against friction and wear.
More research in progress deals with compatibility issues that might arise. Wong notes that, under certain conditions, dry nitrogen in contact with polymer materials can affect the performance of the polymer when it is rubbed against steel. Adding a lubricant might resolve this problem because nitrogen does not affect lubricants to the same extent that oxygen does. However, she adds, this needs to be verified with further testing.
Still other research is needed to evaluate the effects of various lubricant additives. Some friction modifiers and antiwear additives, for example, work under nitrogen as well as or better than they do under oxygen
(see Figures 4 and 5). However, some (but not all) ashless phosphorus antiwear additives need oxygen to work effectively.
6 Other studies are addressing the chemistry of degradation mechanisms—tribodegradation versus autodegradation, for example. Modeling studies might also be useful for delineating reaction mechanisms under low oxygen concentrations.
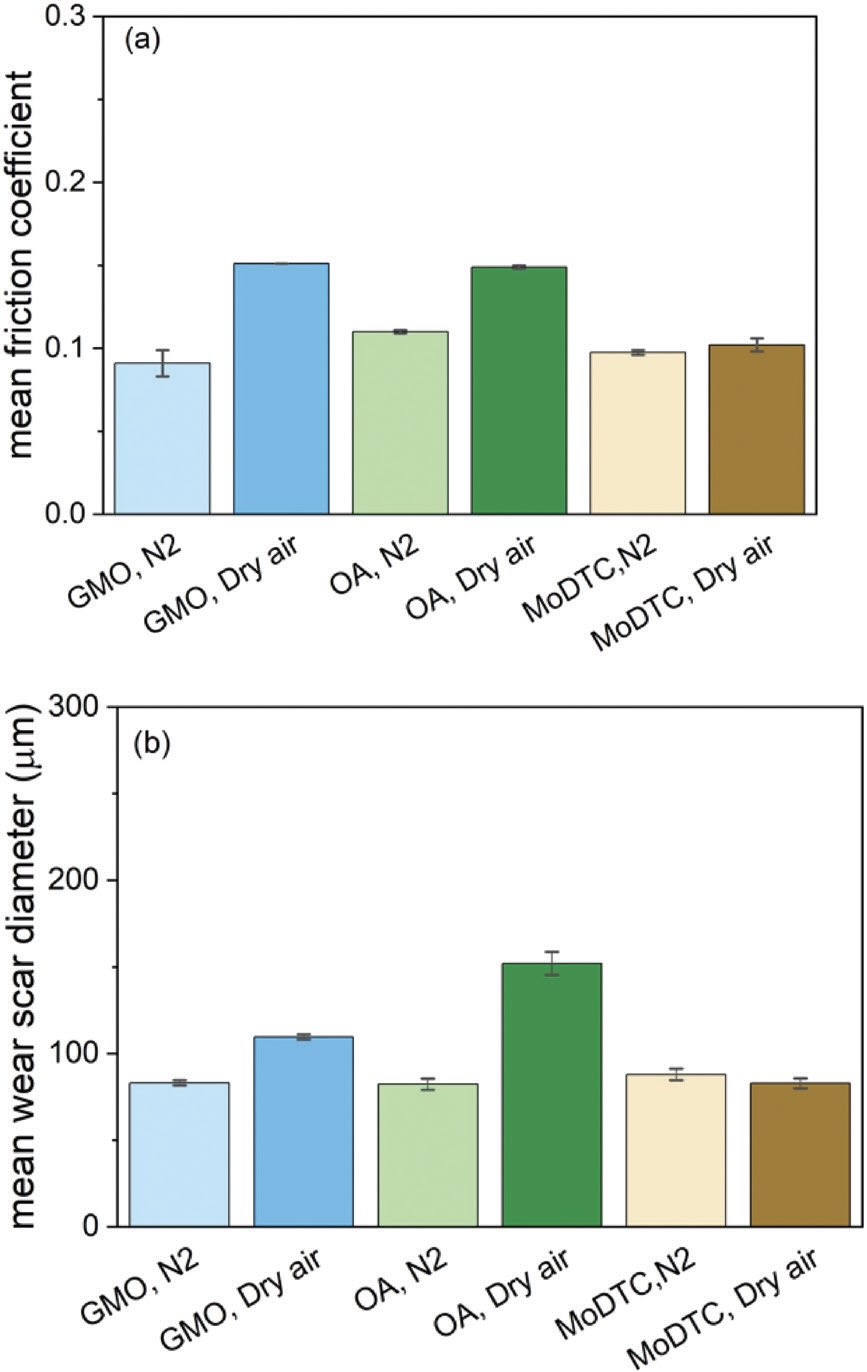 Figure 4. a.) Friction and b.) wear performance of two organic friction modifier additives (glycerol monooleate and oleylamide) and molybdenum dialkyldithiocarbamate in hexadecane at 60°C. Error bars show the range of the values. Figure courtesy of Reference 6, CC BY license.
Figure 4. a.) Friction and b.) wear performance of two organic friction modifier additives (glycerol monooleate and oleylamide) and molybdenum dialkyldithiocarbamate in hexadecane at 60°C. Error bars show the range of the values. Figure courtesy of Reference 6, CC BY license.
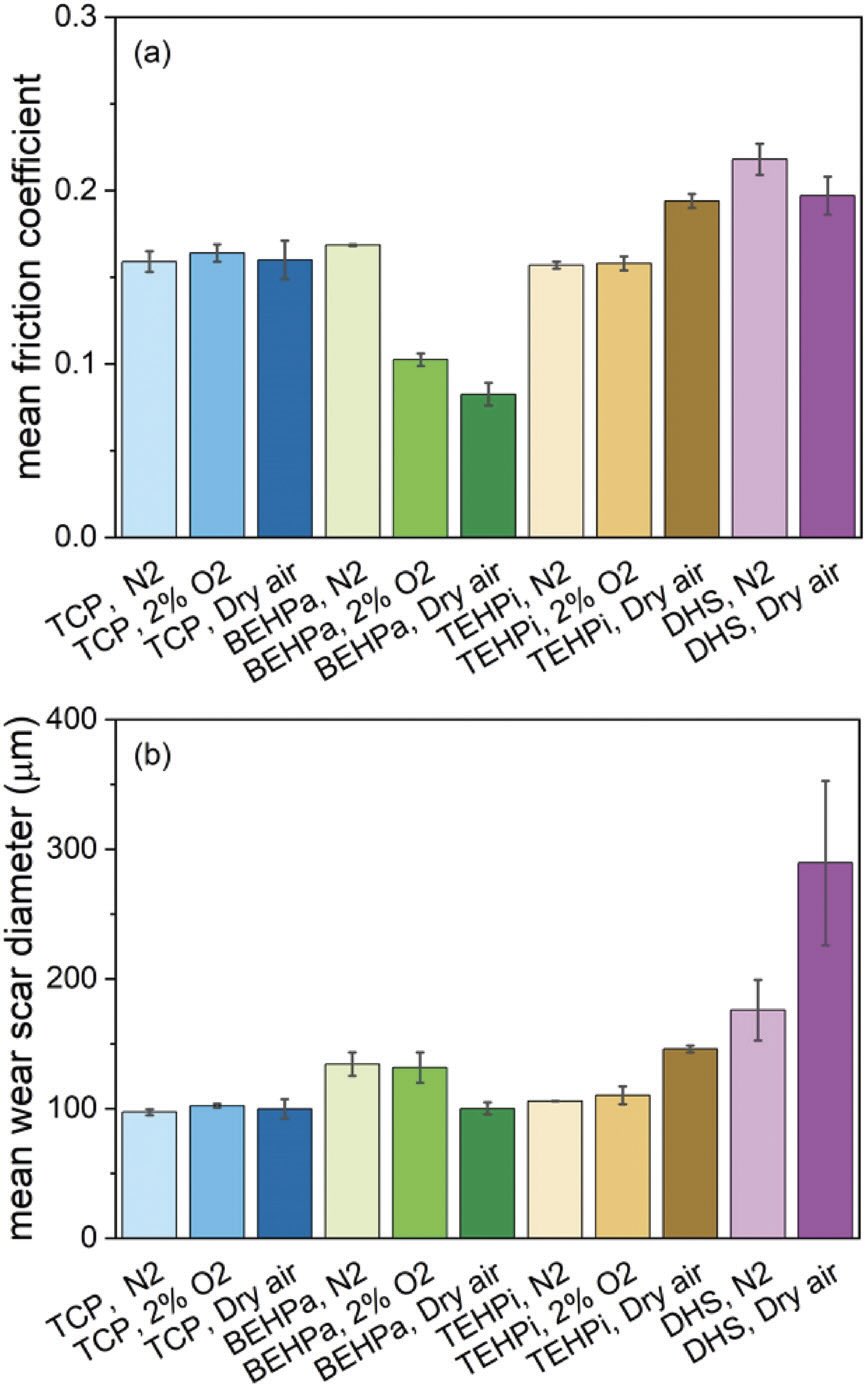
Figure 5. a.) Friction and b.) wear performance of model antiwear and extreme pressure (EP) additives. Error bars show the range of the values. TCP is tricresyl phosphate, BEHPa is bis(2-ethylhexyl)phosphate, TEHPi is tris(2-ethylhexyl) phosphite and DHS is dihexyldisulfide. Figure courtesy of Reference 6, CC BY license.
Spikes notes that the amount of research still to be done could keep several academic groups busy for quite some time. However, he hopes that researchers will not get so absorbed in interesting chemistry problems that they lose sight of the reason for doing this research in the first place. “I would like to see this used in practice,” he says, “either to lubricate systems that get very hot and have very short lives, or to extend life indefinitely.” He foresees regulatory requirements, health and environmental issues and economic factors playing a role in driving applied, goal-oriented research in this area.
From experiment to application
Wong cautions that just preventing oxidation is not sufficient for making a commercially successful product. Formulations that provide superior performance at a good price, as well as helping with sustainability, are more likely to succeed in the marketplace, she says.
Factors lowering the barrier to adoption include the relatively low price, small size, light weight and low power consumption of today’s nitrogen concentrators. One nitrogen concentrator on the market today is small enough to hold in one hand, and it produces one liter per minute of nitrogen with 1% oxygen. The technology is similar to that used in commercial oxygen concentrators, which have gotten even smaller and lighter because of increased demand for portable and home units.
Over the next several years, Pasaribu says, researchers should be able to gain a better understanding of how inerting works with typical lubricant additives. This would enable them to select a group of formulations to use in field trials, which could then be used to plan scale-up efforts. Running lab tests and field trials concurrently could allow each one to inform the other, accelerating the overall process. He notes that although the likely timeline might seem too long for automotive manufacturers, it is a reasonable projection for industrial machinery like turbines, gearboxes, compressors and other systems that use large amounts of liquid lubricants. Several industrial equipment suppliers are aware of this work, Pasaribu says, and they are considering it as a possibility.
In today’s wind turbine gearboxes, for example, the longest oil drain interval warranty is currently 10 years. “Now imagine,” Pasaribu says, “if with this new technology we can extend it even more.” Longer drain intervals decrease the effort and downtime needed to change the lubricant. This, in turn, affects the supply chains that go into lubricant manufacturing, as well as the costs of transporting technicians, supplies and equipment to remote wind farms. Thus, the value added factors in the volume and expense of the lubricant, the criticality of the equipment and the cost and frequency of planned maintenance. Also, longer, more reliable performance could significantly reduce expenses associated with unplanned maintenance, he says. For offshore wind turbines, unscheduled maintenance requires arranging for a boat on short notice and paying emergency rates for a skilled technician to travel to the site and work under harsh environmental conditions. The used oil must be put into containers and transported back to a disposal site. All of these expenses provide a strong motivation to keep the lubricant functioning for as long as possible.
For smaller systems and systems that use grease, it is probably less expensive to replace the lubricant and dispose of the used lubricant than to invest in the energy needed to generate an inert atmosphere, at least for now, Pasaribu says. However, if inerting works well in large, critical applications, EVs and other small-volume applications might follow later. The concept should work. However, he adds, the smaller volumes of lubricant involved and the ease of changing motor and transmission oil makes the economic advantage less clear.
The main challenge, Pasaribu says, is the fragmentation of work being done on the various components needed to make lubricant inerting into a commercial application. “It’s not new knowledge,” he says. “NASA has done this in the 1950s and 1960s.” However, suppliers of nitrogen concentrators don’t focus on lubricant behavior, lubricant formulators aren’t focusing on delivery systems that could reduce the amount of product they sell, and equipment manufacturers aren’t convinced that they should invest in a new technology that is still being proven. “That’s why we need to bring this together,” he says. “In this way, we bring the technology to the customer with enough knowledge so that the customer is willing to say that it makes sense and it’s logical.” He adds that making lubricants last longer is becoming more important in response to environmental legislation and customer demand for sustainability. “If you can quantify it and actually improve the performance of your system, then it becomes something really viable.”
Lubricant suppliers could benefit from this effort as well, Pasaribu says. Major suppliers already advise their customers about how to select and maintain their products. He doesn’t consider nitrogen concentrator suppliers to be his company’s competition. Rather, “They are actually our technology supplier,” he says. Lubricant inerting could be a way of making lubricants more cost efficient and lubricated systems more operationally efficient.
Making the commercial case
For an equipment manufacturer to adopt a new technology like lubricant inerting using a nitrogen concentrator, they need evidence that the technology provides benefits in real-world operations. Hansen explains that manufacturers must run tests on gears and bearings to assess the inerted lubricant’s ability to protect against wear, scuffing and fatigue, as compared with the same lubricant under air. Björklund adds that the cost, space requirements and weight of an inerting system and its effect on energy consumption must also be considered.
Industrial partners could help their academic counterparts by providing data from their own tests. They could also provide their collaborators with test parameters and operating conditions for vehicles and other equipment to ensure that their research results are relevant. However, they must be convinced that the potential benefits are worth the investment of time and resources required to run the tests. Preliminary tests could be performed at a smaller scale and move to full-scale testing and possibly field testing if the results are promising.
Laboratory testing by academic groups has demonstrated the potential of lubricant inerting, but the tests have mainly focused on base oils and simple combinations of additives. Fully formulated lubricants are more complex. The interactions between combinations of additives and base oils (and hardware components) under nitrogen are only beginning to be investigated. Understanding these interactions will be critical to evaluating the potential of inerting technology in real-world applications.
At present, EV lubricant formulations have not been standardized to the same extent as their ICE counterparts, which adds to the complexity of evaluating formulations. However, if research groups can establish parameters and general principles for common formulations and develop test methods for evaluating lubricant performance in the absence of oxygen, this knowledge could be used as a basis for evaluating other formulations.
In short, running lubricated systems under a nitrogen atmosphere could enable much longer lubricant lifetimes, including fill-for-life applications. Systems and components could run at higher temperatures, reducing the need for cooling. A dry, non-oxidizing atmosphere would facilitate the use of ester lubricants, including biobased esters. Reducing the formation of acids and peroxides would help to prevent corrosion in yellow-metal applications like copper motor windings. Antioxidant lubricant additives could be reduced or eliminated without an associated increase in ferrous metal oxidation. And oxidation-driven lubricant viscosity changes and additive depletion could be reduced or eliminated. Potential applications include lubrication systems for industrial gearboxes, compressors, turbines, hydraulics, coolant and transformer systems, wind turbines, EVs and aerospace applications.
6 Is lubricant inerting an idea whose time has arrived? We may find out soon.
REGISTRATION
1.
Loomis, W. R., Townsend, D. P. and Johnson, R. L. (Sept. 1969), “Lubricants for inerted lubrication systems in engines for advanced aircraft,”
NASA Technical Note D-5420, https://ntrs.nasa.gov/api/citations/19690026410/downloads/19690026410.pdf.
2.
Farfan-Cabrera, L. I., Lee, S., Skowron, S. and Erdemir, A. (May 2025), “Enhancing lubrication of electrified interfaces by inert gas atmosphere,”
ASME Journal of Tribology, 147, 051104-1. DOI: 10.1115/1.4066649.
3.
Farfan-Cabrera, L. I., Lee, S., Skowron, S. and Erdemir, E. (May 2025), “Enhancing lubrication of electrified interfaces by inert gas atmosphere,”
J. Tribol., 147 (5): 051104,
https://doi.org/10.1115/1.4066649.
4.
Nyberg, E., Hansen, J. and Minami, I. (2020), “Lubrication concept evaluated for geared actuators under starved conditions,” Proceedings of the 45th Aerospace Mechanisms Symposium, NASA Johnson Space Center, pp. 255-260. NASA/CP-20205009766.
https://ntrs.nasa.gov/api/citations/20205009766/downloads/45th%20AMS%20Proceedings-Final.pdf.
5.
Osso, C. (March 6, 2025), “Fuel cell system with inert gas separator and aircraft having a fuel cell system,” U. S. Patent Application U.S. 2025/0079486 A1.
6.
Zhang, J., Leung, M. C. Y., Wong, J. S. S. and Spikes, H. (Feb. 5, 2025), “Lubricant inerting,”
Tribology Transactions, 68 (1), pp. 180-193,
https://doi.org/10.1080/10402004.2024.2448803.
7.
Zhang, J., Bolle, B., Wong, J. S. and Spikes, H. A. (2024), “Influence of atmosphere on carbonaceous film formation in rubbing, metallic contacts,”
Tribol. Lett., 72 (4),
https://doi.org/10.1007/s11249-023-01801-9.
Nancy McGuire is a freelance writer based in Albuquerque, N.M. You can contact her at nmcguire@wordchemist.com.