HIGHLIGHTS
•
Single-atom catalysis involves the placement of isolated single atoms on a solid support surface and has the potential to improve reaction kinetics.
•
A current hydrogenation study using single-palladium-atom catalysts on metal oxide nanoparticles of various sizes demonstrated conversions from acetylene to ethylene of greater than 90%.
•
In the case of a 1.9 nanometer zinc oxide semiconducting support, the activity of this single-atom catalyst was approximately 46 times greater than a benchmark catalyst.
Hydrogenation is an important reaction needed to manufacture such important lubricant raw materials as Group II and III base stocks and polyalphaolefins (PAOs). In the case of Group II and III base stocks, hydrogenation serves as a finishing step to removing undesirable components from both base oils. For PAO, hydrogenation is an important step in the reduction of oligomers.
Hydrogenation is conducted through the use of a precious metal catalyst such as palladium or platinum. While effective, the high cost of these catalysts and their consumption can increase the cost of conversion of a raw material into a finished product.
One approach that is under evaluation to improve reaction kinetics is the use of single-atom catalysis. Zhenxing Feng, associate professor of chemical engineering at Oregon State University in Corvallis, Ore., says, “Single-atom catalysts are literally isolated single atoms placed on a solid support surface that have the ability to catalyze a specific reaction. Their use represents a trend to move toward efficient catalysis with full metal atom utilization. This property is extremely important for catalysts because they operate on surfaces. The surface of larger-sized, metal catalysts such as bigger platinum particles is where surface metal atoms are only effective. Atoms in the interior are not used for catalysis and are, in effect, wasted.”
Single-atom catalysts were evaluated in catalytic converters in a study that was discussed in a previous TLT article.
1 Researchers positioned single platinum atoms in certain locations on a cerium oxide support to determine performance in the oxidation of carbon monoxide. Benefits were seen by placing platinum atoms at cerium oxide edge sites and embedding them in the cerium oxide lattice, so they were not on the surface but not in the interior. Catalytic activity increased by as much as 3.5 times higher than adsorbed platinum. This result led researchers to estimate that the content of this precious metal could decrease by at least 60% leading potentially to comparable performance at a lower cost.
The catalytic activity of a single atom is also dependent upon the metal oxide support, which hosts the metal atom. Feng says, “A single-atom catalyst by itself is simply not stable as there is risk of degradation and also agglomeration with other atoms leading to a significant reduction in activity. To move forward in exploiting the benefits of single-atom catalysts, work needs to be done to better understand how they will interact with specific metal oxide supports in various processes where catalysts are used such as oxidation and reduction. A strong interaction may not necessarily be advantageous if the single-atom catalyst cannot adsorb and desorb the adsorbates involved in a specific reaction. A weak interaction may also not be beneficial as the single-atom catalyst would not gain the benefit of its relationship with the metal oxide support.”
Feng and his colleagues, including Professor Junling Lu at the University of Science and Technology of China, conducted a study to better understand the interactions between a single-atom catalyst and a variety of metal supports.
Hydrogenation of acetylene
The researchers synthesized 34 single-atom palladium catalysts and evaluated them on 14 types of semiconducting supports. Initially, semiconducting supports were prepared from a series of metal oxide particles including zinc, cobalt, nickel, titanium and gallium. Feng says, “There are a large number of supports including those based on alumina and silica. These are relatively inexpensive but may not provide the interactions necessary with a precious metal single-atom catalyst. The selection of semiconductor metal oxides was done because these materials have defined and tunable molecular orbital structures to interact with individual palladium atoms.”
Metal oxide supports with varying nanoparticle sizes were grown on spherical silica substrates. Single palladium atoms were then added to the metal oxide nanoparticles by using atomic layer deposition
(see Figure 3). The series of single-palladium-atom catalysts were then evaluated by hydrogenation of acetylene to produce ethylene. Feng says, “Hydrogenation of acetylene is a very important industrial reaction, and palladium has been found to be a good catalyst for this process.”
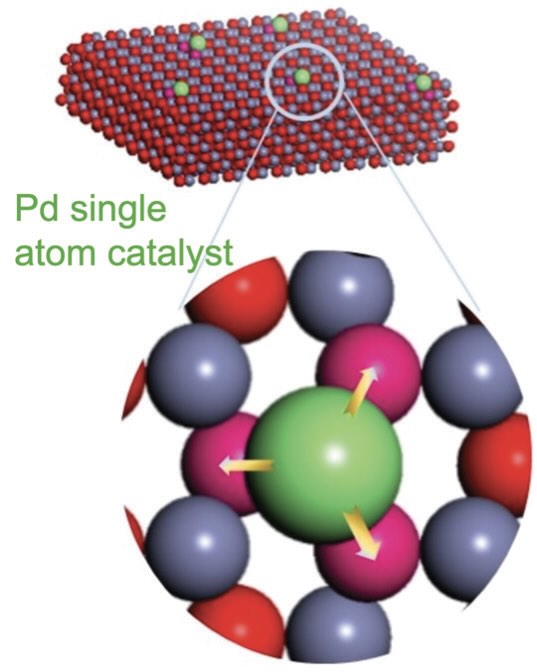 Figure 3. Placement of a single palladium (Pd) atom on a metal oxide catalyst support leads to an increase in catalytic activity in the hydrogenation reaction. Figure courtesy of Oregon State University.
Figure 3. Placement of a single palladium (Pd) atom on a metal oxide catalyst support leads to an increase in catalytic activity in the hydrogenation reaction. Figure courtesy of Oregon State University.
For most of the catalyst/semiconducting support systems, conversions of greater than 90% were realized. The researchers determined that catalytic activity and stability increased as the size of the metal oxide particles decreased. Feng says, “This phenomenon was especially noted for zinc oxide where the activity of a 1.9 nanometer semiconducting support was approximately 46 times greater than a benchmark palladium-silver, single-atom catalyst supported on silicon oxide. Equally important was the small sized oxide catalyst did not show a decrease in activity for at least 100 hours. Palladium single atoms on larger-sized zinc oxide supports (8.5 nanometer and bulk zinc oxide) displayed significant deactivation. The bulk catalyst suffered from agglomeration.”
Heterogeneous catalyst performance is optimized if the Sabatier principle is followed. Feng says, “The Sabatier principle states that how strongly the catalyst adsorbs to the reactant will indicate whether the reaction can be successfully completed. Too strong of an interaction means that the reactant remains bound to the catalyst, hampering the conversion into the desired product and subsequent desorption. Too weak of an interaction is an indication that the catalyst cannot convert the reactant to the desired products. The ideal property for a catalyst is in-between these two extremes.”
This aspect of catalyst performance is critical because Feng indicates that activation of the hydrogen molecule by initial disassociation of the bond between the two atoms followed by adsorption on the catalyst is the rate determining step for hydrogenation.
The reduction in the size of the metal oxide semiconducting support appears to be a significant reason for an increase in the activity of the single-atom palladium catalyst. Feng says, “Using modeling we determined that a reduction in the particle size of the metal oxide elevates the position of the lowest unoccupied molecular orbital so that it better interacts with the highest occupied molecular orbital of the palladium atom. The result is better catalyst activity and durability.”
Future work will involve gaining a better understanding of the mechanism for how the metal oxide semiconducting support interacts with single-atom catalyst. Feng says, “We will change the amount of single-atom catalysts present on the metal oxide support and also evaluate other catalyst candidates such as a palladium-copper alloy."
Additional information can be found in a recent article
2 or by contacting Feng at
zhenxing.feng@oregonstate.edu.
REFERENCES
1.
Canter, N. (2023), “Improving the efficiency of catalytic converters,” TLT,
79 (5), pp. 18-19. Available at
www.stle.org/files/TLTArchives/2023/05_May/Tech_Beat_I.aspx.
2.
Shi, X., Wen. Z., Gu, Q., Jiao, L. Jiang, H., Lv, H., Wang, H., Ding, J., Lyons, M., Chang, A., Feng, Z., Chen, S., Lin, Y., Xu, X., Du, P., Xu, W., Sun, M., Li, Y., Yang, B., Zhang, T., Wu, X. and Lu, J. (2025), “Metal support frontier orbital interactions in single-atom catalysis,”
Nature, 642, E25.