Meet the Presenter
This article is based on a webinar titled Elucidating the Atomic-scale Mechanisms responsible for Adhesion, Friction and Tribochemistry using Computer Simulations. Hosted by the American Society of Mechanical Engineers’ (ASME) Tribology Division and presented by Judith A. Harrison on March 6, 2024, the session explored atomic-scale mechanisms of friction, adhesion and tribochemistry in covalent systems. Courtesy of STLE, this article captures the core insights from this ASME-organized event. For more technical content from the ASME Tribology Division Webinar Series, visit the ASME Tribology Division’s website at www.asme.org/get-involved/groups-sections-and-technical-divisions/technical-divisions/technical-divisions-community-pages/tribology-division.
Judith A. Harrison has been a professor of chemistry at the U.S. Naval Academy for more than 30 years and is currently the chair of the Chemistry Department. She received her doctorate degree from the University of New Hampshire, performing her graduate work in the area of gas-phase reaction dynamics. Before joining the faculty of the Naval Academy, she was an American Society of Engineering Education postdoctoral associate at the Naval Research Laboratory working with Carter T. White, Donald W. Brenner and Richard J. Colton. She has held visiting scientist positions at the University of Pennsylvania and the Johns Hopkins University. Her research has focused on the theoretical examination of atomic-scale processes, such as indentation, friction, wear and tribochemistry of hydrocarbon systems using molecular dynamics (MD) simulations. More recently, she has been examining the relationships between molecular structure and thermophysical properties in Navy-relevant fuels and supercritical fuel properties. She has published approximately 90 technical papers, she presented the Burt L. Newkirk Lecture at Rensselaer Polytechnic Institute and has been a keynote speaker at the STLE annual meeting. She has won several awards including the Naval Academy’s Research Excellence Award, the Department of the Navy’s Superior Civilian Service Award, the George Braude Award, she is a two-time recipient of the Department of the Navy’s Meritorious Service Award and is a Fellow of both the American Vacuum Society and STLE. She is currently a member of the American Chemical Society, Sigma Xi and American Society of Mechanical Engineers (ASME).
You can reach her at jah@usna.edu.

Judith A. Harrison
KEY CONCEPTS
• Friction and adhesion at the atomic level impact covalent systems, particularly silicon-silicon interactions. These mechanisms are critical for microelectromechanical systems (MEMS) and semiconductor devices.
•
Chemical terminations, such as hydroxide and hydrogen, affect adhesion forces and material wear. Also, factors such as roughness, steric effects and prior wear history influence the formation of interfacial bonds, impacting both adhesion and friction.
•
Revelations regarding these factors enable the design of more reliable MEMS and semiconductor devices by tailoring surface chemistry and textures to control friction and adhesion.
This article is based on a webinar by Judith A. Harrison with the U.S. Naval Academy and the Tribology Division of the American Society of Mechanical Engineers (ASME) on atomic-scale mechanisms of friction, adhesion and tribochemistry in covalent systems, with a particular emphasis on silicon-silicon and other covalent material interactions. These insights are highly pertinent to microelectromechanical systems (MEMS) and semiconductor devices, where wear and stiction can pose major obstacles. By investigating the formation and breaking of bonds at the nanoscale level it’s possible to highlight strategies for mitigating mechanical failures in advanced technologies. See Meet the Presenter for more information.
The importance of silicon in technology
Silicon is central to a host of modern applications, including computer chips, watches, phones and various MEMS devices. Yet, as devices become smaller, surface interactions become more problematic, often causing components to stick together. This undesirable sticking is referred to as stiction. An example is tiny gears in MEMS devices that become bent against a base surface, rendering the device inoperative. In such circumstances, because of the large surface-to-volume ratio in miniaturized systems, adhesive forces dominate. Additionally, factors such as wear and tribochemical reactions can exacerbate component degradation over time.
Significance of adhesion and pull-off force
Adhesion relates directly to the pull-off force measured in molecular dynamics simulations. The pull-off force provides a convenient proxy for adhesion strength. Specifically, if two perfectly flat surfaces are in contact, one can evaluate the energy change at separation and divide by the contact area to obtain the work of adhesion. In practice, geometric complexities such as curved or rough surfaces complicate the relationship. Despite this, pull-off force remains a valuable measure.
Van der Waals interactions offer a baseline attraction but become overshadowed when covalent bonds form. (Van der Waals interactions are weak, non-covalent forces that arise between atoms due to temporary shifts in electron distribution. They play a crucial role in molecular recognition, protein folding and material properties.) Roughness can either decrease adhesion by lowering the contact area or potentially create unsaturated sites where bonding is more likely. Nonetheless, the primary contributor to adhesion in covalent materials is bond formation in systems with unsaturated surface sites. When surfaces create covalent links, the force required to separate them rises markedly, largely due to the contributions to the pull-off force arising from the rupture of the covalent bonds.
Molecular dynamics insights into bond formation
Regarding specific simulations performed using molecular dynamics (MD) with reactive potentials, one example is modeling silicon-silicon nanocontacts with hydroxide terminations. Harrison and coworkers employed MD simulations to bring a tip into contact with a substrate, hold it at constant load and then retract it, while tracking the force-separation behavior. By visualizing the atoms and bonding through color coding, it became apparent that material transfer sometimes occurred for certain levels of hydroxide surface terminations, indicating that covalent bonds had formed and then ruptured, pulling portions of one contacting surface onto the other.
At low hydroxide (-OH) coverages, direct silicon-silicon and silicon-oxygen-silicon bonding was more prevalent, resulting in higher pull-off forces and greater material exchange
(see Figure 1). In contrast, at higher hydroxide coverages there are fewer unsaturated sites and more steric hindrance, reducing the opportunity for tight contact required for bond formation between contacting bodies and thus lowering pull-off forces.
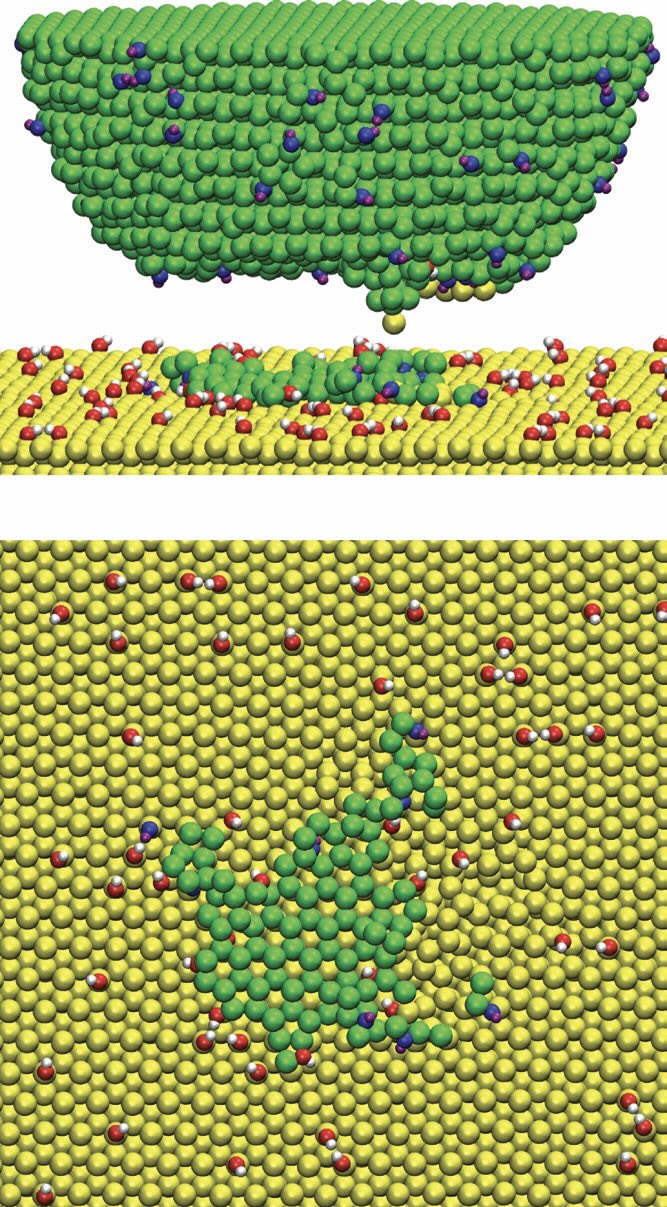 Figure 1. Snapshots of an MD simulation of an -OH terminated Si tip and substrate (with 10% termination) after the tip has been brought into contact with the surface and removed. The upper panel is a side view of the tip, and part of the substrate while the lower panel is looking down on the surface of the substrate after the tip is retracted. Si, O and H atoms that originate in the tip are represented as green, blue and purples spheres, respectively. Si, O and H atoms from the substrate are represented as yellow, red and white spheres, respectively. Figure courtesy of Schall, J. D., Morrow, B. H., Carpick, R. W. and Harrison, J. A. (2024), “Effects of –H and –OH termination on adhesion of Si–Si contacts examined using molecular dynamics and density functional theory,” Langmuir, 40 (9), pp. 4601-4614, https://doi.org/10.1021/acs.langmuir.3c02870.
Figure 1. Snapshots of an MD simulation of an -OH terminated Si tip and substrate (with 10% termination) after the tip has been brought into contact with the surface and removed. The upper panel is a side view of the tip, and part of the substrate while the lower panel is looking down on the surface of the substrate after the tip is retracted. Si, O and H atoms that originate in the tip are represented as green, blue and purples spheres, respectively. Si, O and H atoms from the substrate are represented as yellow, red and white spheres, respectively. Figure courtesy of Schall, J. D., Morrow, B. H., Carpick, R. W. and Harrison, J. A. (2024), “Effects of –H and –OH termination on adhesion of Si–Si contacts examined using molecular dynamics and density functional theory,” Langmuir, 40 (9), pp. 4601-4614, https://doi.org/10.1021/acs.langmuir.3c02870.
Interestingly, intermediate hydroxide concentrations yielded a variety of outcomes, sometimes showing higher pull-off forces and other times showing lower pull-off forces. This variability can be explained by minor shifts in the tip’s position or orientation, which could permit intimate contact in some trials but not in others. Recent work has revealed that this behavior is intimately related to the structure and orientation of the surface hydroxide groups. Additionally, silicon-oxygen bonds were stronger than silicon-silicon bonds, leading the latter to preferentially break when surfaces were separated.
Hydrogen terminations
Simulations where differing amounts of hydrogen terminations were present on both a tip and a substrate were also explored. Because hydrogen is smaller and lacks bulky steric effects, the resulting adhesion patterns diverged from those observed with hydroxide at intermediate terminations. While certain configurations of the tip alignment influence pull-off forces, hydrogen terminations generally did not produce abrupt changes in pull-off forces observed with hydroxide terminated silicon surfaces. The pull-off force decreased monotonically as hydrogen termination was increased. Hydrogen-terminated systems also showed that bond formation was a major determinant of pull-off forces particularly when unsaturated surface sites were present.
Similar principles apply to other material systems, such as diamond-like carbon (DLC), crystalline diamond and ultrananocrystalline diamond. In simulations using DLC, randomly placed unsaturated surface sites allowed for covalent bonding during contact. Repeated indentations in the same region occasionally resulted in varied pull-off forces, highlighting the stochastic nature of bond formation. Even if a surface appeared identical, slight differences in approach angles or local configurations meant bonds might or might not form. This randomness made it difficult to predict adhesion strictly from the number of available unsaturated sites. Data correlating the number of tip-substrate bonds formed with measured pull-off forces show a fairly linear relationship yet also reveal significant scatter as the number of unsaturated surface sites was increased (and surface termination decreased).
Friction mechanisms and wear phenomena
Regarding friction, phonon contributions and bond-related contributions can be distinguished in simulations. Essentially phonons are the quantum-mechanical description of lattice vibrations, in the same way that photons describe light waves. Phonon friction arises from vibrational excitations in the lattice, while bond-related friction stems from the repeated formation and rupture of covalent bonds as surfaces slide. One illustration involves self-mated, hydrogen-terminated silicon nanocontacts. Under some coverage conditions, the system underwent minimal wear, as surface hydrogen prevented extensive bonding between the tip and the substrate. At low surface terminations, hydrogen atoms were worn away, causing silicon-silicon bonding that increased both wear and friction. Over time, the tip’s apex lost atoms progressively, first losing hydrogen and then shedding silicon clusters as higher numbers of interfacial bonds were formed.
Importantly, friction can remain relatively modest in fully terminated or smooth interfaces where phononic effects dominate but escalates considerably once strong covalent bonds come into play. An example is fully terminated crystalline diamond-on-diamond friction, where no wear is observed. By examining vibrational energy in particular bonds, it is clear that friction corresponds to atomic stick-slip events accompanied by local energy spikes that quickly dissipate through the lattice. Lower in the diamond structure, these vibrations were less pronounced, indicating that frictional energy spread outward from the interface. A demonstration with DLC showed how sites without hydrogen could create repeated bond formations and breakages, causing temperature increases at the interface.
Friction at the atomic scale
Controlling friction and adhesion in nanoscale applications relies on understanding the impacts of multiple parameters
(see Summary of Adhesion and Friction). Steric effects, termination species, surface coverage, contact geometry, roughness, temperature and load can all shift whether surfaces bond heavily or remain largely separated. Bond strength also factors in, as demonstrated by the difference between silicon-oxygen and silicon-silicon bonds. Furthermore, factors such as sliding history or prior wear can expose new reactive sites by removing passivating layers, changing how subsequent contacts behave. All of this underscores the complexity of designing surfaces that maintain the desired friction and wear over many cycles.
Summary of adhesion and friction
•
Pull-off forces in covalent materials are directly related to the number of covalent bonds broken while breaking contact. This is a significant contribution to adhesion.
•
The number of bonds formed during contact is a function of contact point, number and type of unsaturated sites, steric constraints, roughness, sliding history and the stochastic nature of bond formation. All of these influence the number of bonds that must be broken during tip-surface separation.
•
In the absence of wear, the friction of covalent materials is dominated by vibrational excitation and dissipation of energy via phonons.
•
Friction is directly related to the number of interfacial bonds formed and broken during sliding. This is the largest contributor to friction in many covalent systems. Dissipation of energy via the excitation of phonons still occurs as shown in the DLC films simulations. Intrafilm reactions can also happen.
There has been much research and related publications linking simulation outcomes with experimental verifications. Joint efforts that combine MD models with atomic force microscopy sliding or tapping-mode experiments help confirm that many of the predicted behaviors align with experimentally observed data. This synergy provides reassurance that, although MD often deals with timescales and system sizes smaller than many real-world systems, the fundamental mechanisms can still be extrapolated to practical engineering solutions.
Tribochemistry and strategies for controlling adhesion and friction
Ultimately, these atomic-scale revelations improve understanding of how best to engineer surfaces of covalent materials, including those for MEMS, integrated circuits and protective coatings in mechanical systems. Through carefully chosen chemical modifications or surface textures, it becomes feasible to reduce stiction or manipulate friction, thereby extending the lifespan of devices.
In certain circumstances, strong bonding might be advantageous, because it could anchor wear-resistant layers in place. In others, passivation with a chosen termination chemistry can maintain low adhesion, preventing damage from contact events. Consequently, research bridging nanoscale interactions and macroscale device reliability is a priority.
Building on more than two decades of exploration, researchers continue to steadily advance knowledge in tribology and surface science, uncovering how friction, adhesion and tribochemistry intersect in covalent systems. By linking empirical observations with computational insights, they continue to clarify the fundamental processes behind wear, stiction and mechanical fatigue.
These discoveries align with broader trends in miniaturization and allow engineers to incorporate tribological design principles at the earliest phases of product development. For instance, when designing MEMS resonators or micro-engines, material choices and surface treatments can drastically influence device longevity.
Collaboration across disciplines such as chemistry, physics, materials science and mechanical engineering make it possible to tackle tribological challenges effectively. By encouraging open dialogue and data sharing, researchers can accelerate progress toward robust, next-generation technologies that depend on reliable nanoscale interfaces.
Future direction
Atomic-scale mechanisms of friction, adhesion and tribochemistry in covalent materials are far from trivial. These mechanisms involve complex interactions between surface chemistry, mechanical forces and properties, thermal conditions and random molecular events. Yet, by systematically employing MD simulations, laboratory experiments and theoretical modeling, scientists can increasingly predict how surfaces will behave under a wide range of conditions, including varying loads, temperatures, material type, and type and degree of surface termination.
The promise of lowering friction, preventing stiction and controlling wear represents a major step forward in fields including semiconductor manufacturing, surface coatings, sensors, MEMS and precision instrumentation. As a result, ongoing research in these domains is likely to yield innovative, application-specific solutions that harness the unique properties of covalent systems while mitigating the risks associated with friction, adhesion and tribochemistry at the nanoscale level.
By sharing data, refining simulation potentials and continuously updating experimental protocols, the field of nanoscale tribology can advance at a faster pace. New insights will shape a generation of devices that merge high performance with exceptional reliability.
In cutting-edge applications, it might be beneficial to promote strong covalent bonding with the express intent of enhancing wear resistance. Examples include certain protective coatings, where robust interfacial bonds can anchor a hard, durable layer in place. However, balancing such bonding with the need to avoid stiction remains an engineering challenge.
By refining the chemistry at critical interfaces, it is possible to achieve partial bonding that delivers mechanical stability without causing parts to seize or jam. Researchers are currently exploring nanocomposite films and functionalized surfaces that exhibit this balance, offering both lubrication and structural cohesion at the nanoscale.
Another area of interest involves investigating electronic contributions to friction in conductive covalent materials. While phonon and bond-related mechanisms are often central, certain materials under high loads or extreme temperatures may experience nontrivial electronic effects for particular compositions. These could involve a charge transfer at the sliding interface or energy dissipation through electronic excitations. Studies combining
in situ spectroscopy with nanoscale tribology experiments may help illuminate whether such electronic phenomena could be harnessed for friction control.
Engineers remain focused on how these fundamental discoveries can revolutionize device architecture. By integrating specialized coatings directly into fabrication processes, or by applying them as post-production treatments, developers could significantly reduce frictional losses. Additionally, sensor components operating at high frequencies could benefit from minimized damping, extending operational life and stability.
Although progress in this field demands considerable effort, the rewards are substantial and include smaller, more efficient devices with unparalleled reliability. As the world increasingly relies on miniaturized electronics, sensors and mechanical components, these tribological insights will be crucial to sustaining advances in technology.
Conclusion
There is no single solution for all covalent systems. Instead, success depends on tailoring surface termination, material composition, geometry and environmental parameters for a specific context. This calls for close collaboration between theorists, computational scientists and experimentalists who can validate proposals in realistic conditions.
Currently, bridging the gap between molecular insights and practical engineering designs is a primary objective in tribology. It will ensure that the potential of covalent materials is fully realized. Experts anticipate that ongoing research will yield strategic guidelines for commercial adaptation, enabling entire industries to harness fined-tuned atomic-scale interactions for reduced downtime, elevated performance and lower energy consumption.
Going forward, there is a need for continued research to refine simulation tools, incorporate more realistic conditions and develop robust machine-learning models that can predict tribological performance under diverse scenarios.
For Further Reading
1.
Schall, J. D., Morrow, B. H., Carpick, R. W. and Harrison, J. A. (2024), “Effects of –H and –OH termination on adhesion of Si–Si contacts examined using molecular dynamics and density functional theory,”
Langmuir, 40 (9), pp. 4601-4614,
https://doi.org/10.1021/acs.langmuir.3c02870.
2.
Schall, J. D., Milne, Z. B., Carpick, R. W. et al. (2021), “Molecular dynamics examination of sliding history-dependent adhesion in Si–Si nanocontacts: Connecting friction, wear, bond formation, and interfacial adhesion,”
Tribology Letters, 69, 52,
https://doi.org/10.1007/s11249-021-01431-z.
3.
Milne, Z. B., Schall, J. D., Jacobs, T. D. B., Harrison, J. A. and Carpick, R. W. (2019), “Covalent bonding and atomic-level plasticity increase adhesion in silicon–diamond nanocontacts,”
ACS Applied Materials & Interfaces, 11, 43, pp. 40734-40748,
https://doi.org/10.1021/acsami.9b08695.
4.
Bernal, R. A., Chen, P., Schall, J. D., Harrison, J. A., Jeng, Y.-R. and Carpick, R. W. (2018), “Influence of chemical bonding on the variability of diamond-like carbon nanoscale adhesion,”
Carbon, 128, pp. 267-276,
https://doi.org/10.1016/j.carbon.2017.11.040.
5.
Ryan, K. E., Keating, P. L., Jacobs, T. D. B., Grierson, D. S., Turner, K. T., Carpick, R. W. and Harrison, J. A. (2014), “Simulated adhesion between realistic hydrocarbon materials: Effects of composition, roughness, and contact point,”
Langmuir, 30, 8, pp. 2028-2037,
https://doi.org/10.1021/la404342d.
6.
Schall, J. D., Gao, G. and Harrison, J. A. (2010), “Effects of adhesion and transfer film formation on the tribology of self-mated DLC contacts,”
Journal of Physical Chemistry C, 114, 12, pp. 5321-5330,
https://doi.org/10.1021/jp904871t.
7.
Gao, G. T., Mikulski, P. T., Chateauneuf, G. M. and Harrison, J. A. (2003), “The effects of film structure and surface hydrogen on the properties of amorphous carbon films,”
Journal of Physical Chemistry C, 107, 40, pp. 11082-11090,
https://doi.org/10.1021/jp034544+.
8.
Harrison, J. A. and Brenner, D. W. (1994), “Simulated tribochemistry: An atomic-scale view of the wear of diamond,”
Journal of the American Chemical Society, 116, 23, pp. 10399-10402,
https://doi.org/10.1021/ja00102a006.
Note: Agencies supporting research in this field include the Air Force Office of Scientific Research, the National Science Foundation, the Office of Naval Research and the Naval Academy Research Office.
Jeanna Van Rensselar heads her own communication/public relations firm, Smart PR Communications, in Naperville, Ill. You can reach her at jeanna@smartprcommunications.com.