HIGHLIGHTS
•
Ethylene oxide, a key intermediate used to manufacture different types of lubricant additives and base stocks, is produced by an energy intensive process at a low conversion rate.
•
A single-atom alloys strategy was used to prepare a better catalyst that contains nickel atoms on a silver substrate.
• A better conversion rate was achieved with the new catalyst while also suppressing secondary ethylene oxide combustion.
Ethylene oxide (see Figure 3) is a chemical intermediate used in the manufacture of different types of lubricant additives and base stocks. For example, ethylene oxide is used to manufacture nonionic emulsifiers that stabilize emulsifiable oil and semisynthetic metalworking fluids. Ethylene oxide is combined with propylene oxide at various ratios to produce a wide range of polyalkylene glycols (PAGs) that lubricant formulators use as base stocks in specific lubricant applications.
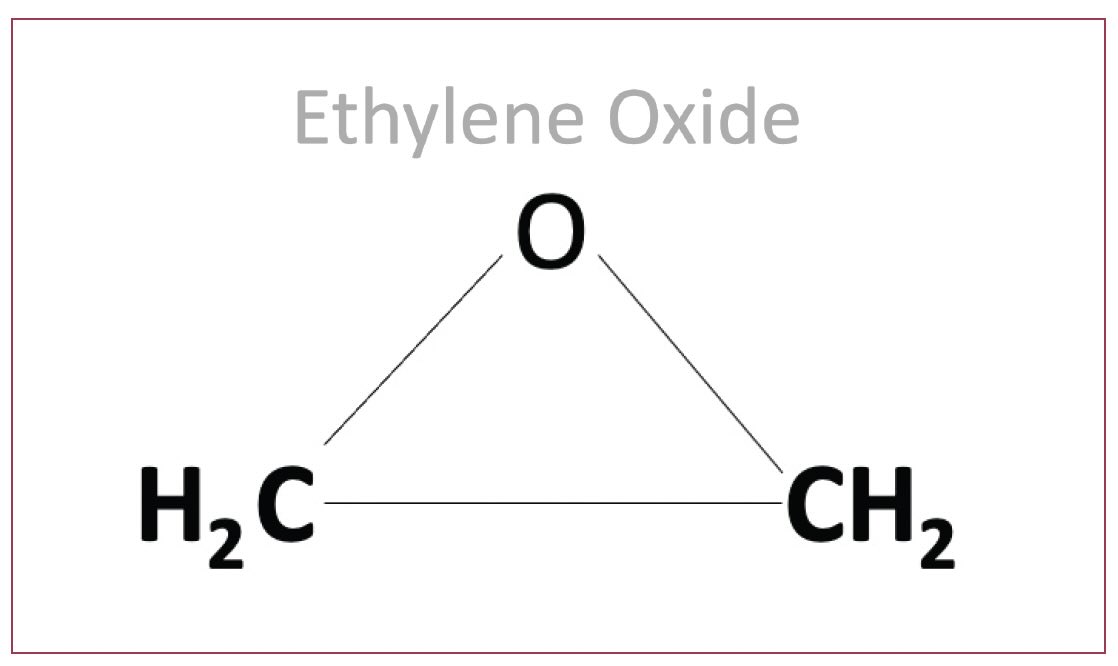 Figure 3. The structure of ethylene oxide, an intermediate used to produce a large number of lubricant additives and base stocks, is shown. Figure courtesy of Chemical Solutions.
Figure 3. The structure of ethylene oxide, an intermediate used to produce a large number of lubricant additives and base stocks, is shown. Figure courtesy of Chemical Solutions.
The lubricant field is starting to demonstrate the value of its products in reducing emissions, and as a consequence saving energy and reducing operating costs for end-users. As part of this process, lubricant suppliers are working to better understand their product carbon footprints. This includes determining emissions generated in the processing of raw materials such as ethylene oxide. More than $40 billion worth of ethylene oxide is produced annually, generating roughly 40 megatons of carbon dioxide.
Matthew Montemore, associate professor and Robert and Gayle Longmire Early Career Professor in Chemical Engineering at Tulane University in New Orleans, La., says, “Ethylene oxide is currently produced from ethylene and molecular oxygen. A silver/alpha-aluminum trioxide heterogeneous catalyst is required to produce this chemical intermediate. The challenge in preparing ethylene oxide in this manner is that the more thermodynamically favored process is to convert ethylene oxide and oxygen into carbon dioxide and water.”
A related concern is that ethylene oxide is very reactive making it difficult to produce and keep stable under the processing conditions. For this reason, the per-pass conversion of ethylene to ethylene oxide is maintained at only 10%-15%. This requirement means that the reactants (ethylene oxide and oxygen) must be recycled.
Montemore says, “Recycling is an energy intensive process because ethylene oxide must be separated from the raw materials. In addition, about 10% of the product mixture is carbon dioxide which adds to the emissions generated. To address this challenge, finding an approach that minimizes the direct combustion of ethylene and the secondary combustion of newly formed ethylene oxide with oxygen is crucial.”

One option used commercially to improve the reaction yield is the introduction of a promoter to enhance the ability of the catalyst system to selectively produce ethylene oxide. Montemore says, “Chloride in the form of an alkyl chloride has been found to be effective when co-fed with the raw materials. Ethylene oxide selectivity improves by approximately 25% at low ethylene conversions. Chloride appears to depress primary and secondary combustion which improves the effectiveness of the silver catalyst.”
Chloride’s negative issues involve corrosion and potential environmental concerns. Other promoters such as alkali metals and oxyanions of transition metals produce only a slight increase in moving the reaction to ethylene oxide production and away from combustion.
Modification of the existing silver catalyst with a different transition metal has now improved selectivity and conversion to ethylene oxide.
Nickel
Montemore and his colleagues used a strategy based on so-called single-atom alloys (SAA), which successfully identified catalysts for other applications. He says, “Our approach was to consider the well-known silver catalyst as a host material and to identify a second metal that can be used as a dopant. Each atom of the second metal will be isolated and embedded into the host.”
In using density functional theory to calculations on selective oxidation, the researchers found that nickel is an excellent fit as the second metal. Montemore says, “Nickel has two characteristics that work well for the production of ethylene oxide. The metal will moderately bind oxygen atoms but will also promote disassociation of molecular oxygen almost without having to overcome an energy barrier.”
Initial work with nickel atoms on a silver substrate involved surface-science studies of oxygen activation on silver single crystal surfaces doped with 1% nickel. The single crystal was exposed to oxygen at low temperatures, heated up and mass spectroscopy was used to determine what volatile species came off. This substrate was a flat surface and the process evaluated under vacuum conditions. These experiments, performed by graduate students Elizabeth Happel and Laura Cramer, under the supervision of Professor Charles Sykes at Tufts University, give deep insight into the surface structure and reactivity.
Dissociated oxygen was not detected in experiments with the control silver catalyst. With nickel-doped silver, mass spectroscopy found that molecular oxygen was coming off the surface. Montemore says, “Nickel atoms initially became embedded in the silver upon initial exposure. Once oxygen was introduced, nickel atoms migrated to the surface of the host substrate, silver, and were positioned to potentially promote conversion of ethylene to ethylene oxide.”
The final phase of research was to prepare highly diluted nickel-silver nanoparticle alloys and compare their ability to produce ethylene oxide versus the base silver/alpha-aluminum oxide catalyst. These experiments were performed by graduate student Anika Jalil under the supervision of Professor Phil Christopher of the University of California, Santa Barbara. Particle size proved to be an important parameter in this study. Montemore says, “The average diameter for silver nanoparticles was approximately 70 nanometers because smaller and larger particles can be less stable or active. Various nickel to silver ratios were evaluated and we found that a ratio of 1:200 nickel to silver atomic ratio produces a 25% selectivity increase for ethylene oxide which is comparable to the promoter effect seen with chlorine.”
This result was achieved when the catalyst was exposed to 10% ethylene and 10% molecular oxygen at temperatures of 473 K, 498 K and 523 K. A selectivity benefit was seen at the highest reaction temperature which suggests that the presence of nickel also serves to suppress secondary ethylene oxide combustion.
An additional 10% benefit in selectivity was found when the researchers combined the nickel, silver catalyst with chlorine. Montemore says, “Future work will hopefully entail conducting more industrial studies to better understand the potential of the nickel-silver catalyst. We will also try to better understand how this catalyst achieves better selectivity.
The greater selectivity identified in this study means that there is a lower need to recycle the raw materials leading to a more sustainable process that uses less energy. Additional information can be found in a recent article
1 or by contacting Montemore at
mmontemore@tulane.edu.
REFERENCE
1.
Jalil, A., Happel, E., Cramer, L., Hunt, A., Hoffman, A., Waluyo, I., Montemore, M., Chirstopher, P. and Sykes, E. (2025), “Nickel promotes selective ethylene epoxidation on silver,”
Science 387 (6736), pp 869-873.