HIGHLIGHTS
•
Removing hydrophobic materials that are oil soluble from wastewater is challenging.
•
A metal-organic cage based on iron (II) as the metal and six ligands was effective in removing six steroids from wastewater.
•
The reason for their removal is the six steroids all have the right size and right three-dimensional orientation to fit in the metal-organic cage.
Developing techniques for more effectively waste treating water prior to discharge to Publicly Operated Treatment Works (POTWs) and purifying water so that it is suitable for use in industrial applications continues to be challenging. Water remains a precious resource, and new approaches are required to cost effectively purify it for consumer and industrial use.
Per- and polyfluoroalkyl substances (PFAS) have emerged as a significant health and safety issue. These materials are exceptionally stable over a long period of time, leading to the need for finding techniques for removing them from water and decomposing them. In a previous TLT article,1 a process known as hydrodynamic cavitation was used to remove and degrade PFAS from wastewater. During hydrodynamic cavitation, wastewater is subjected to a reduction in pressure when moving through a restrictive area. This action leads to the formation of bubbles that grow and then collapse, generated a significant amount of energy that is able to degrade several PFAS compounds up to 55% over a short period of time.
Hydrophobic materials including those designated as fats, oils and greases are difficult to remove from wastewater. One approach that has been under evaluation is the use of water-soluble coordination cages. Jack Wright, doctoral candidate and study lead researcher at the University of Manchester in Manchester, UK, says, “Metal-organic cages are one example that has been under evaluation in a wide range of applications including catalysis. These substances are prepared by finding the right metal and surrounding it with chelating organic ligands. The problem has been identifying water-soluble cages that contain the right sized void cavity to trap and isolate specific hydrophobic substances.”
Current strategies for producing water-soluble cages involve the use of kinetically inert metal ions or retroactively inducing water solubility by using anion exchange to convert metal-organic cages soluble in solvents such as acetonitrile into water-soluble materials.
A class of substances similar to metal-organic cages is metal-organic frameworks (MOFs). Dr. Imogen Riddell, Royal Society Research Fellow at the University of Manchester, makes a distinction between the two types of complexes. She says, “MOFs are polymeric, porous materials consisting of metal clusters and organic ligands. They are useful in applications such as gas separation. But they are not designed to be used in aqueous environments because they are not truly soluble in water.”
In contrast, metal-organic cages are discrete complexes with specific structures that have demonstrated water solubility. Riddell and her colleagues have now identified a specific metal that, when combined with ligands containing sulfonate functionality, is water soluble and can remove hydrophobic materials.
M4L6 cage
The researchers determined that the type of metal-organic cage that would meet the requirements of having a void cavity with sufficient volume (527 cubic angstroms) to trap a targeted hydrophobe can be prepared with four metal atoms and six ligands (M
4L
6 cage). Riddell says, “Based on past experience, we believed that iron would meet these requirements because this metal atom in the +2 (II) valence state forms a tetrahedron with the desired stoichiometry.”
To identify a ligand that has the potential to be water soluble, the researchers turned to sulfonate-based derivatives which have also demonstrated compatibility with iron (II). Specifically, the researchers determined that such a ligand consisting of a disodium sulfonate salt can be prepared in a two-step procedure. A subsequent reaction with iron (II) sulfate tetrahydrate, 2-formylpyridine, in water produces an Fe
4L
6 tetrahedron with the structure shown in Figure 1.
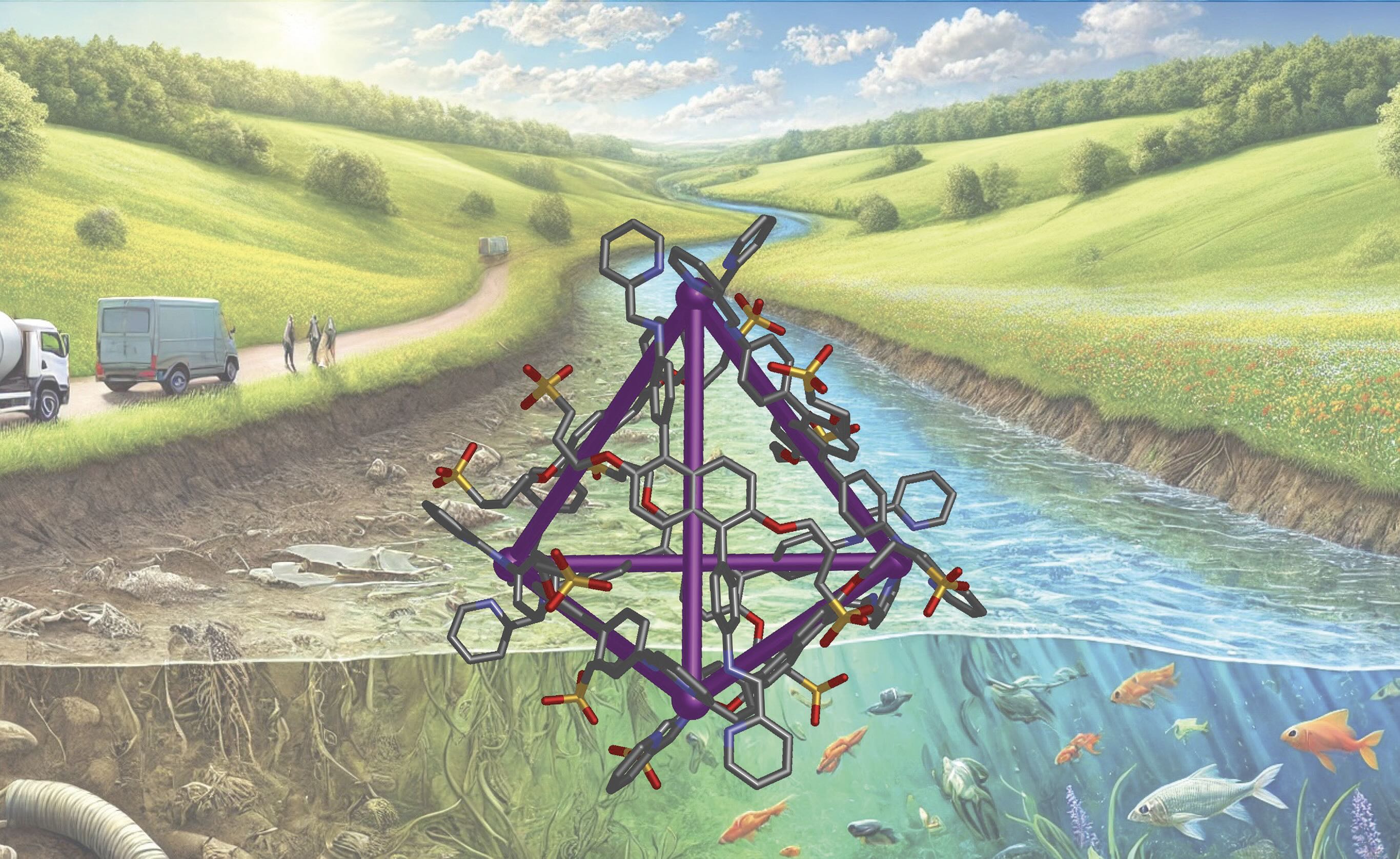 Figure 1. A schematic of a metal-organic cage in the shape of a tetrahedron was able to remove specific steroid hydrophobes from wastewater. Figure courtesy of the University of Manchester.
Figure 1. A schematic of a metal-organic cage in the shape of a tetrahedron was able to remove specific steroid hydrophobes from wastewater. Figure courtesy of the University of Manchester.
Initial testing of the capability of the water-soluble metal-organic cage to bind molecules focused on evaluation of the hydrophobic materials progesterone, ethinylestradiol and tonalide. Riddell says, “Prior to conducting our testing, we believed these molecules exhibited the right size to fit into the cage. These molecules were also selected because they are widely found in wastewater.”
The water-soluble metal-organic cage was able to bind the three hydrophobic materials as determined by
1H nuclear magnetic resonance spectroscopy and mass spectroscopy analysis. This favorable result led to further testing with other steroids including testosterone, estradiol and cholesterol. All three molecules were trapped in the metal-organic cage.
But griseofulvin, an antifungal medicine and fluticasone propionate, a common inflammatory drug, with similar polycyclic ring structures to progesterone did not bind. Riddell says, “The reason these molecules do not bind is because they are either less hydrophobic than other guests or they have a three-dimensional orientation that is not compatible with the void cavity within the water-soluble metal-organic cage.”
Future work will concern developing a procedure for removing the hydrophobic molecule from the water-soluble metal-organic cage in a regeneration process. Riddell says, “We are trying to achieve this regeneration by exposing the bound metal-organic cage to an organic solvent that will lead to efficient removal of the hydrophobic material.”
The success of this approach should lead to the development of additional water-soluble metal-organic cages that can be tailored to trap specific hydrophobes based on designing compatible void cavities. This may include finding ways to trap fats, oils and greases that are common effluents discharged by industrial facilities.
Additional information can be found in a recent article
2 or by contacting Riddell at
imogen.riddell@manchester.ac.uk.
REFERENCES
1.
Canter, N. (2025), “New approach for PFAS removal from wastewater,” TLT,
81 (2), pp. 14-15. Available at
www.stle.org/files/TLTArchives/2025/02_February/Tech_Beat_II.aspx.
2.
Wright, J., Whitehead, G., Knapp, E. and Riddell, I. (2025), “Encapsulation of hydrophobic pollutants within a large water-soluble [Fe4L6]4- cage,”
Cell Reports Physical Science, 6 (2), 102404.