HIGHLIGHTS
•
A [3] catenane was synthesized that produces unidirectional motion in an electrochemical cell without generating any waste.
•
The unidirectional motion is achieved through a series of oxidation and reduction reactions.
•
Synthesis of the [3] catenane is a relatively easy process.
A good deal of attention is being paid to the development of battery electric vehicles that are powered by motors on the macroscopic scale. The automotive industry is working extensively to transition to this technology from internal combustion-powered engines.
Concurrently, research has been underway for some time to develop molecules that can move on the molecular level. A previous TLT article
1 documents efforts made to produce a motorized nanocar. A multiple-stage synthesis process was required to synthesize the molecules incorporated into nanocars that are 3-by-4 nanometers in size. Among the key components were alkynyl groups used as axles that can spin freely at room temperature and fullerene wheels that permit smooth rotation of the molecular wheel. Molecular motion was induced through the use of a solar engine or by using a scanning tunneling microscopy (STM) tip.
Dr. Long Zhang, postdoctoral fellow in the department of chemistry at Northwestern University in Evanston, Ill., says, “Efforts have been made to develop molecular motors that can demonstrate unidirectional motion on a molecular scale. Candidates have been reported as single- molecule electric motors. The problem is that a STM tip is required to move these molecular motors. Other approaches for inducing movement include extreme conditions such as the use of ultrahigh vacuum.”
In an effort to find an alternative approach, Zhang and his colleagues, including Nobel Laureate Sir Fraser Stoddart, professor in the department of chemistry at Northwestern University, decided to focus on designing a molecular motor based on a catenane that can be powered by electricity while in solution. If the right type of electrochemistry is utilized, such an environment should facilitate unidirectional movement. Zhang says, “Catenanes consist of two or more intertwined rings that cannot be separated without breaking covalent bonds.”
Initial work focused on producing a [2] catenane where one cyclobis (paraquat- p-phenylene) (CBPQT
4+) ring encircles a larger loop that contains a bis(4-methylenephenyl) methane (BPM) unit. When placed in solution under an external voltage, the [2] catenane did not show a directional motion. Instead, the CBPQT ring switches between the BPM unit and a second component in the loop known as a viologen (V).
Unidirectional motion powered by electricity was accomplished after the researchers modified the catenane structure.
[3] catenane
Unidirectional motion was achieved through the introduction of a second CBPQT
4+ ring to form a [3] catenane that is approximately two nanometers wide
(see Figure 1). This molecular motor consists of two CBPQT
4+ rings that encircle the loop.
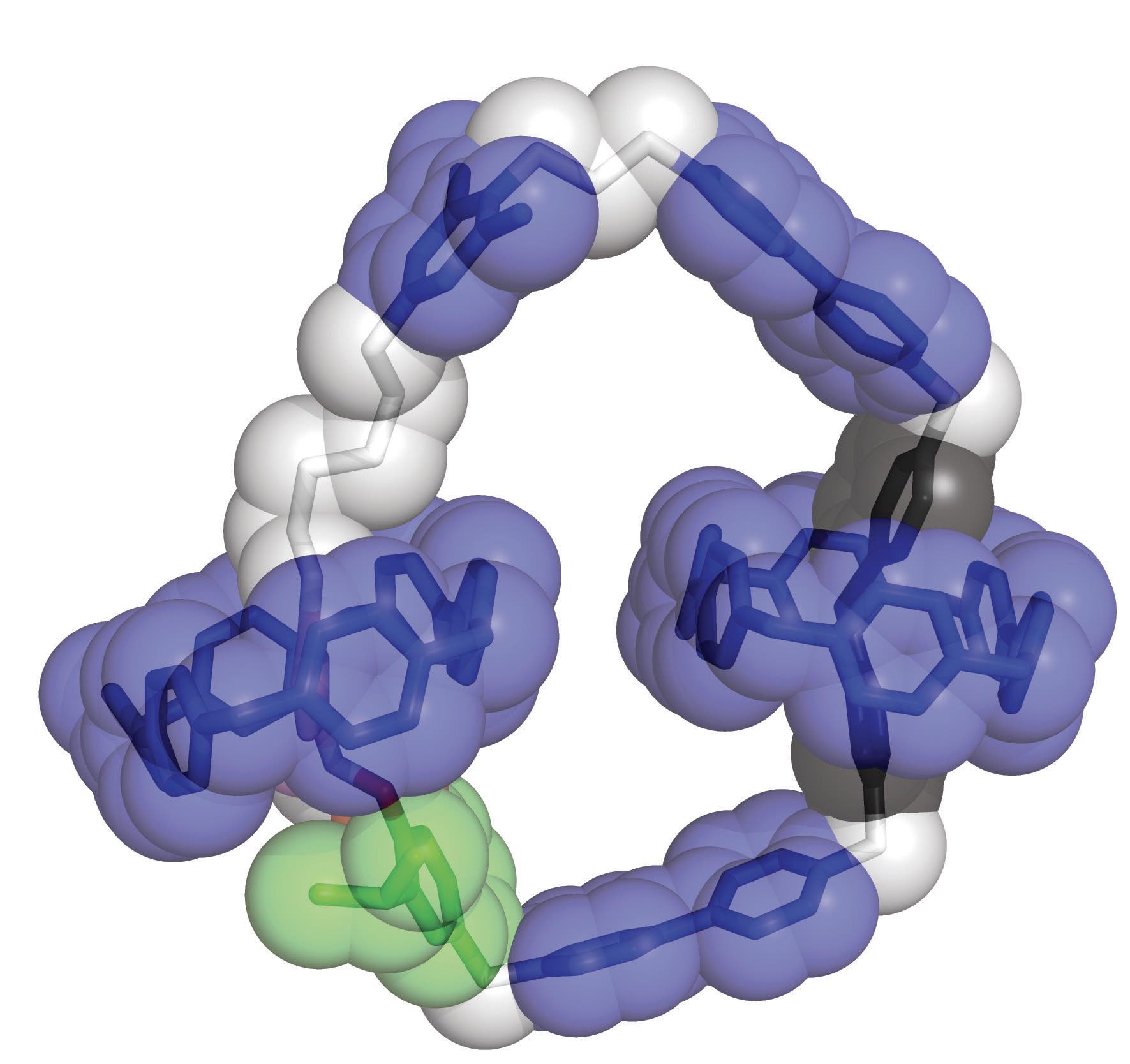 Figure 1. A [3] catenane that is approximately two nanometers wide is able to demonstrate unidirectional motion through the movement of two CBPQT4+ rings that encircle a larger loop. Figure courtesy of Northwestern University.
Figure 1. A [3] catenane that is approximately two nanometers wide is able to demonstrate unidirectional motion through the movement of two CBPQT4+ rings that encircle a larger loop. Figure courtesy of Northwestern University.
By application of an oscillating voltage, the two CBPQT
4+ rings traverse the circular loop through a series of oxidation and reduction reactions. Zhang says, “When the [3] catenane is in an oxidized state, the two CBPQT
4+ rings are in the 3 and 9 o’clock positions on the loop. They are both positively charged, which leads to their separation by Coulombic repulsion. Introduction of six electrons in a reduction reaction facilitates the movement of the two CBPQT
4+ rings to the 6 and 12 o’clock positions, respectively. Oxidation then removes the six electrons enabling the two CBPQT
4+ rings to move again back to the 3 and 9 o’clock positions on the loop.”
During this redox cycle, the two CBPQT
4+ rings completed a 180-degree positional exchange.
The researchers then decided to deuterate one of the CBPQT4+ rings in order to confirm spectroscopically that unidirectional motion had indeed occurred. Zhang says, “We had evidence of motion, but it was difficult to confirm because both CBPQT
4+ rings are chemically identical. By introducing deuterium atoms in place of hydrogen atoms in one of the rings, we were able to show, using 1H nuclear magnetic resonance spectroscopy, that 85% of the molecular motors completed a 180-degree unidirectional cycle.”
The unidirectional motion was conducted in an electrochemical cell that switched between -0.5 Volts and +0.7 Volts. Zhang says, “No byproducts are generated when the process uses an electrochemical cell, which makes operation of the motor sustainable. Movement of the two CBPQT
4+ rings during oxidation and reduction takes a total of 20 minutes. If chemical reagents such as zinc (for reduction) and iminooxidanium (for oxidation) are used, the operation can take a few seconds, but the tradeoff is it will generate waste.”
Zhang indicates that the synthesis of the [3] catenane only requires four steps based on previously described protocols. He says, “This relatively easy process means that the molecular motor can be produced in large quantities.”
Future work will involve modification of one of the rings through the introduction of a heteroatom such as sulfur. Zhang says, “This modification may allow for the [3] catenane to become attached to an electrode such as gold and through motion, influence the surface and ultimately do some useful work.”
Additional information on the molecular motor can be found in a recent article
2 or by contacting Zhang at
long.zhang@northwestern.edu.
REFERENCES
1.
Canter, N. (2006), “Developing a motorized nanocar,” TLT,
62 (8), pp. 10-11.
2.
Zhang, L., Qiu, Y., Liu, W., Chen, H., Shen, D., Song, B. Cai, K., Wu, H., Jiao, Y., Feng, Y., Seale, J., Pezzato, C., Tian, J., Tan, Y., Chen, X., Guo, Q., Stern, C., Philp, D., Astumian, R., Goddard, W. and Stoddart, J. (2023), “An electric molecular motor,”
Nature, 613 (7943), pp. 280-286.