KEY CONCEPTS
•
Conventional emulsions are not sufficiently stabilized with surfactants over the long term.
•
Thin and nanometer-wide planar carbon structures, known as graphene quantum dots, have been developed that demonstrate superior emulsion stability to surfactants in deionized water and in the presence of high levels of electrolytes.
•
The zwitterionic nature of the graphene quantum dots allows the emulsifier to stabilize emulsions over a wide pH range from 3 to 12.
Specific lubricants operate as emulsions in order to combine the beneficial aspects of oil soluble components and water. Two examples are fire-resistant, water glycol hydraulic fluids and water-based metalworking fluids.
The emulsions are stabilized through the use of anionic and nonionic surfactants that reduce interfacial tension between the oil and water layers. A previous TLT article
1 discussed the use of the single bubble/drop test to evaluate the mechanism used by surfactants to stabilize emulsions. In this test, a single bubble/drop is created on a capillary and then moved at a fixed velocity to a predetermined position at the fluid-fluid interface.
The problem with emulsions (such as an oil-in-water [O/W] emulsion) that are commonly used in lubricant applications is stability. Qingsheng Wang, associate professor in the Artie McFerrin Department of Chemical Engineering and holder of the George Armistead ’23 Faculty Fellowship at Texas A&M University in College Station, Texas, says, “Simple O/W emulsions with a liquid in liquid feature that use surfactants are not stable enough. The interfacial tension between the two liquid layers is reduced and the kinetic stability of the emulsion is improved but not over the long term.”
An alternative to the traditional emulsion is known as the Pickering emulsion, which was identified in 1907. Solid particles are used to stabilize a Pickering emulsion by assembling at the oil-water interface. These particles exhibit diameters in the nanometer range resulting in emulsions that an individual can see through.
Initial Pickering emulsions were developed using spherical particles. Better stability has been achieved with plate-shaped particles such as graphene oxide sheets because they display better surface coverage at the interface. But graphene oxide sheets are limited because of particle aggregation that reduces the stability of the emulsion.
Atomically thin and nanometer-wide planar carbon structures known as graphene quantum dots (GQDs) have demonstrated potential as Pickering emulsifiers because of their nanoparticle size, highly tunable surface properties and ease of modifying those surface properties through functionalization. But stable emulsions cannot be formed by GQDs when electrolytes are present in high concentrations.
Wang and his colleagues have now produced GQD derivatives that can be tuned to demonstrate superior emulsification properties over a broad pH range and under high electrolyte treat rates.
Zwitterionic characteristics
The researchers developed GQD derivatives that achieved superior emulsification over a wide pH range and demonstrated better stability in the presence of high levels of electrolytes by synthesizing structures that exhibit both lipophilic and hydrophilic properties. These zwitterionic structures were prepared by pyrolysis of citric acid followed by further functionalization with dodecylamine and dimethylaminopropylamine. The resulting materials are known as zwitterionic graphene quantum dots (ZGQDs).
Wang says, “This process is a facile synthesis that produces nanoparticles functionalized with tertiary amines and carboxylic acid groups.”
Initial studies were conducted using the hydrocarbon dodecane as the oil phase to produce O/W emulsions. The emulsion contained 90 vol% water and 10 vol% dodecane. ZGQDs were added to the water phase at a concentration of 0.05 wt%.
Wang says, “This emulsion remained stable at pH values ranging from 3 to 12. At low pH values, the stability is due to the cationic nature of the tertiary amine and the protonation of the carboxylic groups. At high pH, complete deprotonation of the carboxylic group leads to a stable emulsifier. At neutral pH values, a zwitterion state is found due to the positive charge of the tertiary amine and negative charge of the carboxylic group.”
The emulsions were prepared again in the presence of up to 20% sodium chloride and up to 4% calcium chloride. As shown in Figure 3, stable emulsions are seen with deionized (DI) water, and solutions containing 10% sodium chloride and 2% calcium chloride.
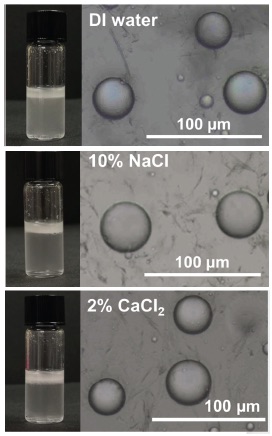 Figure 3. Graphene quantum dots display superior emulsification when used in deionized (DI) water (left image) and in aqueous media with high levels of electrolytes such as 10% sodium chloride (NaCl, center image) and 2% calcium chloride (CaCl2, right image). Figure courtesy of Texas A&M University.
Figure 3. Graphene quantum dots display superior emulsification when used in deionized (DI) water (left image) and in aqueous media with high levels of electrolytes such as 10% sodium chloride (NaCl, center image) and 2% calcium chloride (CaCl2, right image). Figure courtesy of Texas A&M University.
Further work was done replacing dodecane with polystyrene particles prepared through emulsion polymerization. Wang says, “The purpose of this experiment was to observe the polystyrene particle sizes at different pH values. We found that the polystyrene particle sizes were smaller at pH 3 compared to pH 7. The difference in the size of the polystyrene particles is due to the ability of ZGQDs to assembling at neutral pH and then disassembling at acidic pH. This behavior can be used to control the diffusion of molecules through the fluid-fluid interface.”
The researchers investigated molecular diffusion by adding the red dye rhodamine B at 3 wt% to chloroform, which was used as the oil layer with the ZGQDs. Rhodamine B readily diffused into the aqueous layer at acidic pH, but little diffusion was seen at neutral pH. A control experiment showed that rhodamine B readily diffuses into the aqueous layer when no ZGQDs are present at neutral pH.
Wang says, “We have shown that ZGQDs exhibit superior emulsification compared to conventional surfactants. The emulsions formed with ZGQDs are more stable in DI water and even in the presence of electrolytes such as sodium chloride and calcium chloride.”
Wang indicates that future work will be focused on studying the effectiveness of ZGQDs in emulsifying mineral oils. He says, “This initial work represents a proof of concept. We are working to evaluate ZGQDs in different real-world applications.”
Additional information can be found in a recent article
2 or by contacting Wang at
qwang@tamu.edu.
REFERENCES
1.
Canter, N. (2021), “Single bubble/drop test to evaluate foaming and emulsions,” TLT,
77 (2), pp. 16-17. Available
here.
2.
Ma, R., Zeng, M., Huang, D. and Wang, Q. (2022), “Zwitterionic graphene quantum dots to stabilize emulsions for controlled-release applications,”
ACS Applied Materials Interfaces, 14 (5), pp. 7486-7492.