KEY CONCEPTS
•
The presence of carbon dioxide significantly reduces the efficiency of hydroxide exchange membrane fuel cells.
•
A technology has been developed to facilitate the use of hydroxide exchange membrane fuel cells to remove carbon dioxide through modification of an electrochemically driven carbon dioxide separator.
•
Integration of a hydroxide exchange membrane fuel cell into an electrochemically driven carbon dioxide separator was accomplished through shorting the membrane.
Global warming is occurring primarily due to the generation of one specific greenhouse gas (GHG), carbon dioxide emissions. Techniques for removing carbon dioxide through the use of carbon capture are under development but have their limitations.
Dr. Brian Setzler, research assistant professor in chemical and biomolecular engineering at the University of Delaware in Newark, Del., says, “Current approaches for carbon dioxide removal do not exhibit adequate performance for our applications. Examples include the use of aqueous alkaline solutions of potassium hydroxide and solid sorbents of calcium and other metal oxides that produce the resulting carbonates when exposed to carbon dioxide. Reversible solid sorbents such as molecular sieves or supported amines also can capture carbon dioxide.”
In a previous TLT article,
1 the concept of a carbon capture fuel cell vehicle that can be used in marine shipping was discussed. A hydrocarbon-based fuel can react with oxygen producing carbon dioxide and steam through the use of a solid oxide fuel cell. The carbon dioxide produced is placed in a dual-chamber fuel tank that also can store the fuel. A movable partition in the storage tank can be adjusted to compensate for the decline in the volume of fuel and increase in the volume of carbon dioxide as electricity is generated by the fuel cell.
The main type of fuel cell under development contains a proton exchange membrane, uses platinum-based electrodes and operates under acidic conditions. Hydrogen cations move from the anode to the cathode through a negatively charged membrane.
A promising alternative is the hydroxide exchange membrane fuel cell. Setzler says, “This fuel cell involves the transfer of hydroxide anions from the cathode to the anode through a positively charged membrane. More metal catalyst options are available for use in this fuel cell that are cheaper than platinum. But hydroxide exchange membrane fuel cells struggle with durability and display much lower efficiency in the presence of carbon dioxide. Hydroxide exchange membrane fuel cells operate at an efficiency of 55% in the absence of carbon dioxide. Once carbon dioxide is present, efficiency declines by roughly 20%.”
The reasons carbon dioxide reduces the effectiveness of hydroxide exchange membrane fuel cells is because a significant difference in pH is found between the anode and cathode. Reaction of carbon dioxide with hydroxyl anions results in the formation of carbonate species that reduce the pH of the anode to 10, which is in contrast to the cathode pH of 13.5.
Setzler and his colleagues, including professor Yushan Yan, Henry Belin du Pont chair in chemical and biomolecular engineering at the University of Delaware, have now determined that this affinity for carbon dioxide can be utilized to design a system using a hydroxide exchange membrane fuel cell to efficiently remove carbon dioxide.
 Shorted membrane EDCS
Shorted membrane EDCS
The researchers determined that the affinity of the hydroxide exchange membrane fuel cell for carbon dioxide can be used to develop a technology that exclusively is focused on removing this GHG. They based their effort through modification of an electrochemically driven carbon dioxide separator (EDCS).
Setzler says, “EDCS is a dedicated system for capturing carbon dioxide that uses a hydrogen-powered cell with an anionic-conducting membrane. Carbon dioxide is converted to carbonate and bicarbonate at the cathode and then moves across the membrane to the anode. At this point, carbon dioxide is reformed through reaction with hydrogen producing a concentrated stream of this gas.”
Setzler indicates that the potential to integrate a conventional EDCS into a hydroxide exchange membrane fuel cell is limited due to cost and volume considerations. The researchers found that shorting the membrane to allow electrons to flow through the membrane instead of an external circuit simplifies the device and makes it viable for the fuel cell application.
Dr. Lin Shi, a recent doctoral graduate from the University of Delaware, says, “The shorted membrane EDCS needs no electrical wires, bipolar plates or current collectors and, thus, can be modularized like a typical separation membrane and wind into a compact spiral-wound module.”
A schematic of such a device is shown in Figure 2 and has the capability of filtering large quantities of air in a short period of time. The image is similar to that of a reverse osmosis (RO) membrane used in water treatment. Setzler says, “The design of the shorted membrane EDCS was inspired by the structure of a RO membrane.”
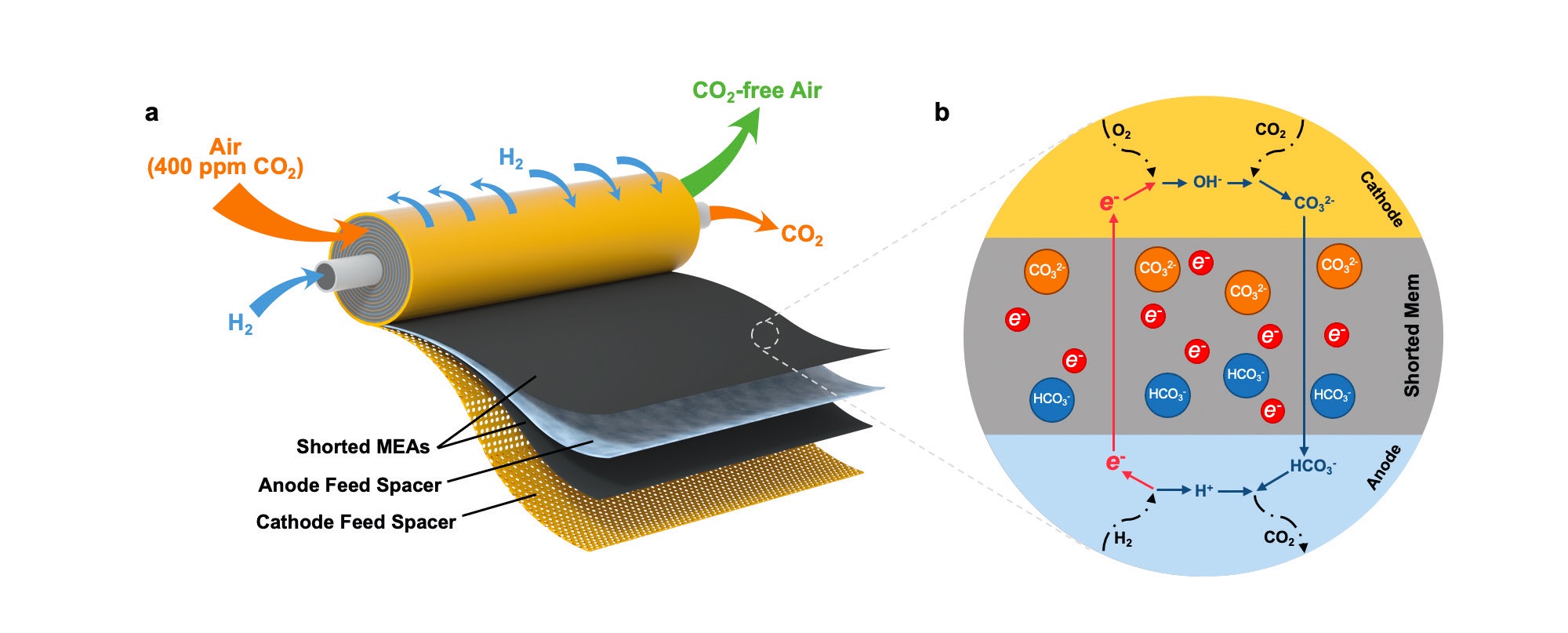 Figure 2. An image of a shorted membrane electrochemically driven carbon dioxide separator is shown on the left and is similar in design to a reverse osmosis membrane. The key to the technology is the flow of electrons directly through a shorted membrane as shown in the schematic on the right. Figure courtesy of the University of Delaware.
Figure 2. An image of a shorted membrane electrochemically driven carbon dioxide separator is shown on the left and is similar in design to a reverse osmosis membrane. The key to the technology is the flow of electrons directly through a shorted membrane as shown in the schematic on the right. Figure courtesy of the University of Delaware.
The researchers evaluated 25 square centimeter single cells and found that the shorted membrane EDCS can remove 99% of the carbon dioxide present in air flowing at a rate of 3 liters per minute. Carbon dioxide removal can exceed 99.9% at lower air flow rates.
Only a small reduction in performance was observed when the shorted membrane EDCS was operated for a 450-hour period. Setzler says, “We will be doing more testing to determine the durability of this device. In addition, we will be making a larger cell and also trying to gain a better understanding of the mechanism that enables efficient removal of carbon dioxide.”
Setzler was asked about the potential for using the shorted membrane EDCS in an internal combustion engine vehicle to remove carbon dioxide. He says, “This approach for carbon dioxide removal is very difficult because the exhaust and high temperature will probably poison the catalyst.”
Additional information on this research can be found in a recent article
2 or by contacting Yan at
yanys@udel.edu.
REFERENCES
1.
Canter, N. (2021), “Carbon capture fuel cell vehicle,” TLT,
77 (11), pp. 12-13. Available
here.
2.
Shi, L., Zhao, Y., Matz, S., Gottesfeld, S., Setzler, B. and Yan, Y. (2022), “A shorted membrane electrochemical cell powered by hydrogen to remove CO
2 from the air feed of hydroxide exchange membrane fuel cells,”
Nature Energy, 7 (3), pp. 238-247.