•
A catalyst based on zinc oxide and copper (I) oxide was able to convert methane to methanol in the absence and presence of water.
•
Higher methanol selectivity was achieved in the water catalyzed reaction.
•
Zinc oxide “islands” sitting on the surface of the copper (I) oxide catalyze the water free reaction while rough areas on the catalyst surface are important in the water catalyzed reaction, which takes place by a different mechanism.
Methane, in the form of natural gas, is found in large quantities, making it an attractive starting material for derivatization into organic derivatives used commercially. A second reason for reacting in methane is to remove it as a greenhouse gas.
Determining a pathway for directly converting methane to methanol appears to be a viable option for reducing the level of methane and using it to make useful derivatives. Methanol is a very useful starting material for a number of important chemical intermediates.
The problem in working with methane is that this molecule is very stable, and finding a way to break its strong carbon-hydrogen bonds is very challenging. In a previous TLT article,
1 researchers developed a more sustainable approach for converting methane to methanol through the use of a bimetallic catalyst based on titanium dioxide and copper (III) oxide under pH neutral conditions. Titanium dioxide catalyzes the formation of methyl from methane while the copper catalyst completes the reaction to form methanol.
Dr. José Rodriguez, senior chemist in the Catalysis: Reactivity and Structure Group at the Chemistry Division of Brookhaven National Laboratory (BNL) in Upton, N.Y., says, “The main challenge for synthesizing methanol from methane is to find suitable experimental conditions at temperatures below 500 K. At any higher temperature, the reaction cannot be controlled.”
To form methanol, methane must be reacted with oxygen in a partial oxidation process. Two competing reactions are possible where methane can be converted to methanol or more completely oxidized to carbon monoxide with the formation of hydrogen as a co-product. In order to maximize methanol formation, the second reaction to carbon monoxide must be minimized.
Rodriguez says, “The important aspect is to find a catalyst that will not oxidize the methyl group formed in the initial step.”
Copper catalysts have been evaluated for this process because an enzyme known as methane monooxygenase is able to use a set of three copper cations to prepare methanol from methane at 300 K. Unfortunately, the process cannot be scaled up for commercial use.
According to Rodriguez, a non-expensive catalyst with a copper-zinc oxide interface appears to be necessary to activate and transform methane. He says, “When other catalysts such as expensive gold or platinum are used in the presence of methane, methanol is not formed.”
Another important component used in the reaction feed to produce methanol from methane is water. While water appears to stop the complete oxidation to carbon dioxide, its presence adds to the complexity of a reaction where large quantities of steam may be present in an industrial process that could potentially be difficult to control.
A new approach has now been developed that can achieve significant methanol formation in the absence of water.
Zinc oxide “islands”
A team of researchers at BNL guided by Rodriguez and Drs. Ping Liu and Sanjaya Senanayake found that reacting methane with a catalyst that is based on zinc oxide and copper (I) oxide produced methanol at a 30% selectivity at 450 K without the use of water. The catalyst was prepared by depositing zinc oxide nanoparticles on a single crystal of copper oriented along the 111 face of the metal under an oxygen atmosphere at a temperature of 600 K. During this process, the surface of the copper single crystal also was oxidized to copper (I) oxide. The resulting catalyst is known as ZnO/Cu
2O/Cu(1 1 1).
Rodriguez says, “We selected zinc oxide because this material, in combination with copper, is a well-known catalyst in the hydrogenation of carbon dioxide that produces methanol. The combination of zinc oxide and copper (I) oxide also is known not to attack methanol, which means that it will not completely oxidize methane to carbon monoxide.”
Scanning tunneling electron microscopy was used to better understand the complex structure of the catalyst. The image shown in Figure 3 identifies three specific types of structures on the copper (I) oxide thin film. Triangular zinc oxide “islands” are found to sit on the surface and become involved with copper (I) oxide in “step” edges
(see A in Figure 3) to catalyze the water free conversion of methane to methanol.
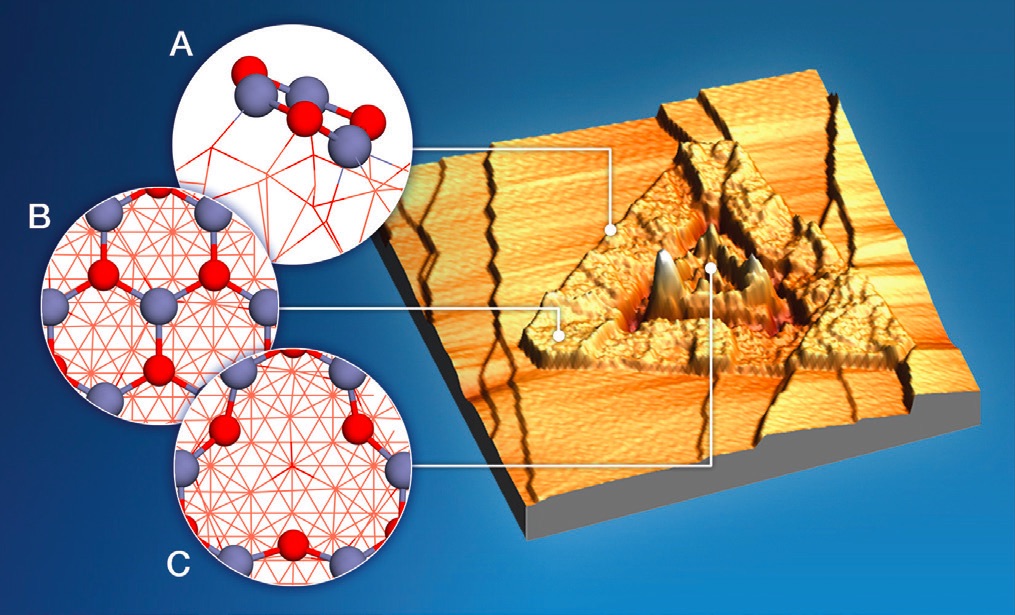 Figure 3. A scanning electron microscope image shown on the right was used by the researchers to better understand the catalyst structure. Triangular zinc oxide “islands,” whose proposed structure is shown in A, are believed to catalyze the water free conversion of methane to methanol. When water is present, rough areas in the catalyst that have the proposed structure shown in C promote the conversion of methane to methanol. Figure courtesy of Brookhaven National Laboratory.
Figure 3. A scanning electron microscope image shown on the right was used by the researchers to better understand the catalyst structure. Triangular zinc oxide “islands,” whose proposed structure is shown in A, are believed to catalyze the water free conversion of methane to methanol. When water is present, rough areas in the catalyst that have the proposed structure shown in C promote the conversion of methane to methanol. Figure courtesy of Brookhaven National Laboratory.
Rodriguez says, “We found that zinc oxide ‘islands’ make multiple sites available for the catalysis. This multiplicity facilitates the reaction.” The BNL researchers also found through theoretical analysis that the local structure of the zinc oxide sites has a major influence on the reaction.
When water is introduced into the reaction, conversion to methanol takes place through a different mechanism. As shown in Figure 3
(see C), the researchers speculate that rough areas on the catalyst surface expedite the conversion of methane to methanol. Rodriguez says, “This section of the catalyst probably contains defects, which have fewer zinc atoms and a more oxygen rich surface. This facilitates the water extraction of the methoxy group.”
The water catalyzed reaction proceeded at a higher methanol selectivity of 80% compared to the anhydrous process. A third section of the catalyst characterized by semi-flat areas
(see B in Figure 3) do not appear to participate in the reaction.
The BNL researchers intend to evaluate different oxides besides zinc oxide to determine if the reaction conversion and selectivity can be improved. Drs. Liu and Senanayake will be heading the theoretical and experimental efforts. Rodriguez says, “In the near future, we will be evaluating highly promising catalysts formed by depositing nanoparticles of zirconium oxide and indium oxide on copper (I) oxide thin films, systems with unique chemical properties.”
Additional information can be found in a recent article
2 or by contacting Rodriguez at
rodrigez@bnl.gov.
REFERENCES
1.
Canter, N. (2021), “Room temperature conversion of methane to methanol,” TLT,
77 (6), pp. 34-35. Available
here.
2.
Huang, E., Orozco, I., Ramirez, P., Liu, Z., Zhang, F., Mahapatra, M., Nemš k, S., Senanayake, S., Rodriguez, J. and Liu, P. (2021), “Selective methane oxidation to methanol on ZnO/Cu
2O/Cu (1 1 1) catalysts: Multiple site-dependent behaviors,”
Journal of the American Chemical Society, 143 (45), pp. 19018-19032.