KEY CONCEPTS
•
A-site and B-site metal cations present in perovskite catalysts were found to affect the performance of the catalyst in the oxygen evolution reaction.
•
pH influences the oxygen evolution reaction as one of the metal cations, scandium, is much more unstable under acidic as compared to basic conditions.
•
Catalysts undergoing extensive surface reconstruction demonstrate higher catalytic activity in the oxygen evolution reaction under acidic conditions.
The major obstacle to producing green hydrogen by splitting water is to identify catalysts that can facilitate the oxygen evolution reaction (OER), which is a four-electron transfer process. A number of different types of metal and oxide catalysts have been evaluated in the OER reaction under acid and alkaline conditions, but most of them have demonstrated limited efficacy.
Zhenxing Feng, associate professor of chemical engineering at Oregon State University in Corvallis, Ore., says, “Metal oxide catalysts have been evaluated, in particular, for use in the OER process. Unfortunately, most of them are unstable under acidic conditions.”
A previous TLT article
1 discussed work Feng conducted to examine the metal oxide thin film model catalyst strontium iridate in the OER. Feng and his colleagues found that this perovskite catalyst is highly active because the structure of strontium iridate changes during the OER. Upon initiation of the reaction, an amorphous layer forms on the surface of the catalyst leading to a change in structure and an increase in effectiveness. Eventually, the catalyst converted from a crystalline to an amorphous square-planar configuration.
This change in catalyst structure is known as surface reconstruction and occurs due to metal atoms present in the catalyst being removed during the OER through a leaching process. Feng says, “Perovskite oxide catalysts have a typical structure designated as ABO
3. A represents a rare earth metal that are needed to stabilize the framework of the crystalline structure of the perovskite oxide. B is the designation for a transition metal or iridium, which is a precious metal. The type of transition metal used is extremely important in determining how active the perovskite oxide is as an OER catalyst.”
Previous studies of perovskite catalysts have shown that A-site metal cations leach more readily than B-site metal cations. But no work has been done to better understand how to control the leaching process to improve catalyst performance, especially on powder materials that can be scaled up for industrial applications. Among the factors is to determine how amorphous surface generation in a specific OER catalyst initiates as the reaction starts due to metal leaching. This will hopefully lead to a better understand of identification of more effective catalysts.
Feng and his colleagues are now reporting a study that demonstrates how controlling both the leaching of A-site metal cations and B-site metal cations in representative perovskite catalysts based on iridium affects catalyst performance under acidic and alkaline reaction conditions.
SSI and SCI
The researchers evaluated the surface reconstruction process using perovskite catalysts designated as SSI and SCI. The molecular formulas for both are shown in Figure 2. Both catalysts utilize strontium (Sr) as the A-site metal cation while the B-site metal cations for SSI and SCI are scandium (Sc) and cobalt (Co), respectively.
Feng says, “We worked with SSI and SCI because both are metal powders and can easily be produced through a solid-state method that involves grinding and calcining the starting materials followed by heating at temperatures above 1,100 C for 12 hours under ambient air conditions. Another benefit is that the structures of both crystalline catalysts can be fully characterized and confirmed through the use of X-ray diffraction.”
Cyclic voltammetry testing was done on both catalysts under alkaline conditions using 0.1 molar potassium hydroxide and under acidic conditions using 0.1 molar perchloric acid. Testing under alkaline conditions shows that the activity of SSI gradually decreases during cycling. In contrast, SCI demonstrates an increase in activity for the first 25 cycles, which then remains constant in subsequent cycling.
Transmission electron microscopy and X-ray photoelectron spectroscopy (XPS) shows that the crystalline structure and composition of SSI does not change during the testing and is reflected in the schematic on the left in Figure 2 that shows an ordered atomic structure. SCI undergoes surface reconstruction that Feng attributes to the slight leaching of cobalt leading to a significant leaching of strontium. The second to the left schematic in Figure 2 shows the amorphous layer at the top, which Feng estimates to be 10 nanometers in depth.
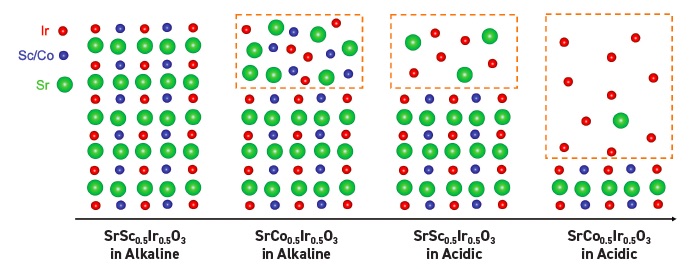 Figure 2. Schematic structures of SSI (the left image and second image to the right) and SCI (second image to the left and the right image) are shown along with their chemical compositions. The left image shows that SSI does not change structure during the oxygen evolution reaction under alkaline conditions while some structure change is found under acidic conditions (second image to the right) leading to the formation of an amorphous layer. Structural change is seen in SCI under alkaline conditions (see second image to the left) but is much more dramatic under acidic conditions (see the right image) where the largest degree of surface reconstruction and the deepest amorphous layer are found leading to higher catalytic activity. Figure courtesy of Oregon State University.
Figure 2. Schematic structures of SSI (the left image and second image to the right) and SCI (second image to the left and the right image) are shown along with their chemical compositions. The left image shows that SSI does not change structure during the oxygen evolution reaction under alkaline conditions while some structure change is found under acidic conditions (second image to the right) leading to the formation of an amorphous layer. Structural change is seen in SCI under alkaline conditions (see second image to the left) but is much more dramatic under acidic conditions (see the right image) where the largest degree of surface reconstruction and the deepest amorphous layer are found leading to higher catalytic activity. Figure courtesy of Oregon State University.
The instability of scandium under acidic conditions is shown in the more pronounced surface reconstruction observed with SSI and SCI. XPS analysis of SSI shows an amorphous layer formed that has a depth of approximately 10 nanometers. But a higher degree of leaching is observed for both strontium and scandium. In fact, almost all of the scandium has been removed leaving an amorphous surface that contains a high percentage of iridium atoms and some scandium atoms
(see the second to the right schematic in Figure 2).
The most extensive surface reconstruction was detected with SCI. Transmission electron microscopy images showed extensive leaching of strontium and cobalt leading to the formation of an amorphous layer that is approximately 50 nanometers deep after 50 cycles. The right schematic in Figure 2 shows the amorphous layer formation where all of the cobalt atoms have left the catalyst.
The large degree of surface reconstruction found with SCI under acidic conditions leads to catalytic activity that is two orders of magnitude greater than currently seen in the OER reaction. Feng says, “By adjusting the type of transition metal present in the B-site, we have found that the performance of perovskite catalysts can be improved. We consider this discovery to be a big step forward in the commercialization of a viable process for producing green hydrogen.”
The researchers are in the process of arranging for partnerships with the private sectors as the first step to achieving commercialization. Feng says, “We also are looking to identify and evaluate better catalysts, particularly ones that do not contain precious metals to reduce costs.”
Additional information can be found in a recent article
2 or by contacting Feng at
zhengxing.feng@oregonstate.edu.
REFERENCES
1.
Canter, N. (2021), “Mechanism of water splitting catalyst,” TLT,
77 (4), pp. 16-17. Available at
here.
2.
Chen, Y., Sun, Y., Wang, M., Wang, J., Li, H., Xi, S., Wie, C., Xi, P., Sterbinsky, G., Freeland, J., Fisher, A., Ager, J., Feng, Z. and Xu, Z. (2021), “Lattice site-dependent metal leaching in perovskites toward a honeycomb-like water oxidation catalyst,”
Science Advances, 7 (50). DOI: 10.1126/sciadv.abk1788.