KEY CONCEPTS
•
Ferrous and non-ferrous metals have different properties making it difficult to develop multi-metal alloys that are effective on both alloy types.
•
Key parameters to consider in selecting a metalworking fluid for multi-metal applications are environmental, health and safety, corrosion (staining) protection performance, fluid longevity, lubricity performance and formulation stability.
•
Picking the right corrosion inhibitors to achieve a balance between minimizing ferrous alloy corrosion and non-ferrous alloy staining is important.
•
Future challenges for metalworking fluid formulators will include selecting additives that cannot only be used in multi-metal alloys but are sustainable and have regulatory profiles that are acceptable globally.
Metalworking fluids are formulated with a large number of components needed not just to provide the essential properties for a specific machining operation but also to minimize microbial growth, minimize foam formation, be stable under hard water conditions and not corrode or stain multiple metal alloys. To meet these challenges, formulators prepare complex fluids that can be one of four types (straight oils, emulsifiable oils, semisynthetic fluids and synthetic fluids).
Due to the uniqueness of each metalworking fluid operation, formulators need to develop and market a large number of products that can add to their operating costs. End-users are looking to consolidate the number of metalworking fluids they are using, which is prompting formulators to determine how to design products that may machine multi-metal alloys that exhibit different properties. It is currently very difficult to identify one metalworking fluid that can effectively machine a ferrous alloy such as carbon steel and a non-ferrous alloy such as wrought aluminum.
One other dimension that is making development of metalworking fluids more difficult involves the ongoing supply chain difficulties faced by formulators. This is coupled with the regulatory challenges limiting additive options that can be used.
The purpose of this article is to discuss the challenges faced by metalworking fluid formulators in developing versatile products that can be used to machine a wide range of alloys and suggest some potential solutions.
Input on the issue was obtained from industry experts who have perspectives from the additive and formulator standpoints.
The following individuals were contacted.
1.
Joe Eldick, Advanced Chemical Concepts
2.
Min Chen, ANGUS Chemical
3.
Harish Potnis, ANGUS Chemical
4.
Kevin Saunderson, BP/Castrol
5.
Stephanie Cole, Clariant
6.
Austin Smith, Clariant
7.
Steven Tang, Colonial Chemical
8.
Bridget Dubbert, Engineered Lubricants
9.
Jim Cancila, Ingevity
10.
William Harwood, Italmatch
11.
Trivendra Kumar, Italmatch
12.
Ron Lemke, Italmatch
13.
David Lindsay, Italmatch
14.
Carl Williams, Italmatch
15.
David Woolliscroft, Italmatch
16.
Dr. Michael Stapels, Kao Chemicals
17.
Andy Yoder, The Lubrizol Corp.
Difficulties in formulating for multi-metal alloy applications
Joe Eldick, vice president of technology for Advanced Chemical Concepts in Rochester, Mich., points out that ferrous and non-ferrous metal alloys have different properties. He says, “Formulators are forced to merge solutions for conflicting requirements into a single fluid, while optimizing overall performance.”
Aluminum alloys are softer and exhibit lower melting points compared to ferrous alloys. Table 1 lists the melting points of typical ferrous and non-ferrous metal alloys and their Brinell hardness numbers (HB).
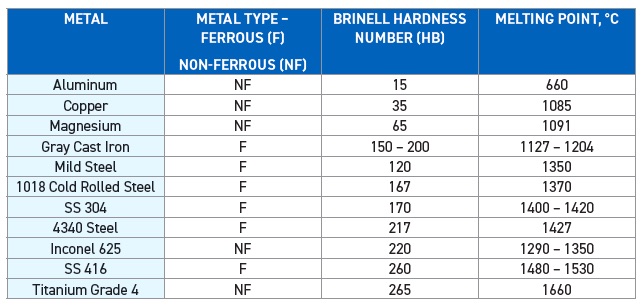 Table 1. Melting points and Brinell hardness numbers (HB) for typical ferrous and non-ferrous alloys are shown. Table courtesy of Advanced Chemical Concepts.
Table 1. Melting points and Brinell hardness numbers (HB) for typical ferrous and non-ferrous alloys are shown. Table courtesy of Advanced Chemical Concepts.
Eldick says, “Machining aluminum alloys is best achieved at low temperature and requires effective boundary lubrication that generates protective films. Due to the differences in chemical properties of aluminum and iron, most boundary lubricants designed for ferrous metals fail to form protective films on aluminum.”
Further details on differences in the physical and mechanical properties of metal alloys can be found in the third edition of “Metalworking Fluids.”
1
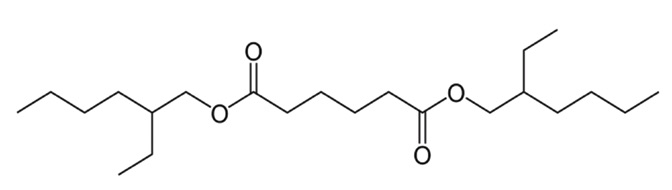 Figure 1. Adipate esters have a structure that contains bidentate bonds, which are very useful as boundary lubricity additives in the machining of aluminum and ferrous metals. Figure courtesy of Advanced Chemical Concepts.
Figure 1. Adipate esters have a structure that contains bidentate bonds, which are very useful as boundary lubricity additives in the machining of aluminum and ferrous metals. Figure courtesy of Advanced Chemical Concepts.
Boundary lubricity additives that are most effective on both alloys contain bidentate or polydentate bonds. Eldick says, “Adipic esters
(see Figure 1) contain this functionality, and they, along with other oxygen containing substances, are effective on both aluminum and ferrous metals.”
STLE member Stephanie Cole, senior formulation chemist for Clariant Corp. in Mount Holly, N.C., indicates there are many obstacles facing a formulator. She says, “A formulator will need to consider the amount of ‘room’ left in a formulation to squeeze in additives. Some additives that provide excellent extreme-pressure (EP) properties on ferrous metals may cause unwanted staining issues on nonferrous metals. For example, active sulfurized additives exhibit excellent high-temperature EP properties but cause staining on yellow metals. A second challenge is preventing corrosion on a wide range of alloys with one fluid. There is only so much room in a formula to add in corrosion inhibitors, and choosing the right ones at the correct dosage can be daunting. Some corrosion inhibitors only work on one type of metal, and those that are multi-metal corrosion inhibitors can have some weaknesses. In some cases, corrosion inhibitors are organic acids and require neutralization to be used, which may
cause corrosion on certain alloys and generate foam.”
STLE member Steven Tang, business manager for Colonial Chemical in South Pittsburg, Tenn., lists the following four challenges facing formulators.
1.
Availability of additives (enabling chemistries) that work equally effectively in both ferrous and nonferrous alloys
2.
Formulation complexity
3.
Formulation cost
4.
Compatibility of enabling chemistries.
Tang says, “Due to the increased demand for higher fuel economy, the use of lightweight metal alloys has been steadily increasing to reduce the weight of automobiles. This means that machining ferrous and non-ferrous alloys with a single metalworking fluid becomes highly desirable. Different metals have different topography and surface properties and, thus, often require different additives to deliver the same types of performance needs, i.e., lubricity, corrosion protection, surface wetting, etc., in their machining processes. To fully accommodate the individual performance demands of different alloys (especially ferrous and non-ferrous alloys) with a single fluid, the resulting formulation will likely be more complicated and more costly. Meanwhile, the compatibility between different additives in the formulation could become a concern from the fluid stability standpoint. Application pH also could become a concern because a high level of alkalinity causes staining of aluminum alloys.”
STLE member William Harwood, global product manager for Italmatch in Cleveland, Ohio, and David Woolliscroft, applicative laboratory specialist for Italmatch in Manchester, UK, both believe that formulators must achieve the proper balance to work with both ferrous and non-ferrous alloys. Harwood says, “A key example is pH, which must be balanced because too low a value will allow a metalworking fluid to be less aggressive to non-ferrous alloys but may lead to corrosion of ferrous alloys where a higher value is preferred. But formulators must be cautious because a pH that is too high may reduce bacterial growth but also may lead to dermatitis. The right level of pH buffering is essential to prolong fluid life.”
Woolliscroft adds, “Formulators will typically produce designated fluids for ferrous applications that may work occasionally on non-ferrous alloys (pending alloy makeup) and vice-versa. The only fluid type that might provide sufficient versatility is the high oil emulsifiable oil, but the main detrimental aspect of this fluid type is lack of sump life.”
Carl Williams, applicative laboratory supervisor for Italmatch in Manchester, UK, states that finding the right type of lubricity additive to work on ferrous and non-ferrous alloys can be problematic. He says, “Non-ferrous metals are softer than ferrous metals and require a more lubricious media that has a much higher ester content to aid surface finish. The reactivity of the two metal alloy surfaces is different meaning that an additive effective on a ferrous alloy may be antagonistic to a non-ferrous alloy. Tighter global regulations (such as the reclassification of European Union [EU] REACH) will further restrict suitable additive candidates.”
Trivendra Kumar, customer technical service manager for Italmatch in Hyderabad, India, feels that finding the right type of passivator is extremely important to develop a metalworking fluid that is effective on multi-metal alloys. He says, “To counteract the high level of amines needed for machining ferrous alloys, a strong passivator that can protect non-ferrous alloys must be included.”
STLE member Dr. Michael Stapels, research and development technical manager for Kao Chemicals in Emmerich am Rhein, Germany, states that different metal alloys have different key requirements making it difficult to optimize metalworking fluids. He says, “Aluminum is theoretically relatively easy to machine but more emphasis is needed on metal chip control compared with ferrous alloys. Finding corrosion control for both ferrous and non-ferrous alloys is difficult due to the conflicting requirements for ferrous and non-ferrous alloys.”
STLE member James Cancila, sales and account manager for Ingevity in North Charleston, S.C., indicates formulators must incorporate a variety of corrosion inhibition strategies to protect against all possible failure modes and work with additives that will not harm the mix of metal being targeted. He says, “The biggest hurdle for formulators will be to produce a multi-metal fluid that is cost competitive with fluids targeting a limited scope of metals. Depending on the metal type, various ‘downstream’ processes (i.e., cleaning, plating, etc.) must be anticipated. The multi-metal formulation must ‘do no harm’ and be compatible with the downstream processing options.”
Andy Yoder, research chemist for The Lubrizol Corp. in Wickliffe, Ohio, feels that a metalworking fluid that can function on multi-metal alloys may not be “best in class.” He says, “Formulators must first understand that lubrication and corrosion protection requirements are different for ferrous and non-ferrous metal alloys. This affects everything from lubricant selection and oil content to concentration requirements and raw material costs. Fully optimizing for one can reduce performance for the other.”
Key parameters
STLE member Harish Potnis, global technical manager – metalworking fluids for ANGUS Chemical in Buffalo Grove, Ill., says, “The key parameters to be considered are the environmental, health and safety profile, corrosion (staining) protection performance, fluid longevity, lubricity performance, foam control, formulation stability and total cost in use. Many of these performance attributes, including fluid longevity and corrosion inhibition, can be achieved by a balanced formulation that uses the right combination of amines and registered biocides.”
Stapels lists the following five parameters that must be taken into consideration in formulating metalworking fluids for multi-metal alloys: material specific lubrication demands, material specific corrosion control, soap formation control, low foam and long-term fluid stability. He says, “On the matter of soap formation control, aluminum fatty acid soaps tend to exhibit more negative characteristics in the overall machining processes compared to metal soaps or typical hard-water ion soaps. Aluminum soaps tend to be more viscous and stickier on surfaces than other metal soaps.”
The negative issues involving aluminum soaps can be overcome through the use of an ether carboxylic acid and a specially designed phosphate ester. Stapels says, “A simple pump test shows how these additives can contribute improved aluminum soap control and increasing emulsion stability to a semisynthetic fluid. For five hours, a 5% metalworking fluid emulsion was circulated while the load of aluminum ions was increased in steps of 10 hardness (178 ppm) until the concentration reached 1,780 ppm. Without the additional emulsifiers, the emulsion splits as shown in the left image in Figure 2. In contrast, the use of these additives stabilizes the emulsion as shown in the right image.
 Figure 2. When added to a formulation, an ether carboxylic acid can improve aluminum soap control, as shown in the emulsion image on the right taken after a semisynthetic fluid was evaluated in a five hour pump test with the water systematically hardened until the concentration reached 1,780 ppm. Without an ether carboxylic acid, the emulsion splits, as shown on the left. Figure courtesy of Kao Chemicals.
Figure 2. When added to a formulation, an ether carboxylic acid can improve aluminum soap control, as shown in the emulsion image on the right taken after a semisynthetic fluid was evaluated in a five hour pump test with the water systematically hardened until the concentration reached 1,780 ppm. Without an ether carboxylic acid, the emulsion splits, as shown on the left. Figure courtesy of Kao Chemicals.
A follow-up experiment was run using the same semisynthetic fluid but replacing all of the emulsifiers with only sodium petroleum sulfonate. After two hours of circulation, the emulsion split, the pump clogged and tacky soaps separated from the fluid surface as shown in Figure 3.
 Figure 3. Repeating the five hour pump test with the same semisynthetic fluid, just containing sodium petroleum sulfonate as the sole emulsifier, leads to emulsion splitting, pump clogging and tacky soaps after two hours. Figure courtesy of Kao Chemicals.
Figure 3. Repeating the five hour pump test with the same semisynthetic fluid, just containing sodium petroleum sulfonate as the sole emulsifier, leads to emulsion splitting, pump clogging and tacky soaps after two hours. Figure courtesy of Kao Chemicals.
Yoder points out that the potential corrosive/staining effect of an additive on both metal types is probably the most important factor. He says, “A second key parameter is that lubricant selection must account for the differences in metal hardness that affect chip formation.”
STLE member Ron Lemke, business development and OEM manager for Italmatch in Cleveland, Ohio, says, “Often multiple lubricity additives must be used, and balancing these competing chemistries is required, so they are optimized for the material and operations being performed.”
STLE member David Lindsay, area sales manager and customer technical manager for Italmatch in Cleveland, Ohio, says, “Staining/corrosion inhibition and microbial control performance must be evaluated with any new metalworking fluid machining new alloy combinations.”
While Harwood is in agreement that esters are beneficial in machining softer non-ferrous alloys and providing good surface finish on the harder ferrous alloys, hydrolytic stability is critical. He says, “The formulator needs to select a specific ester type for a particular multi-metal alloy application that displays minimal hydrolysis at the pH range where the metalworking fluid is operating.”
Cancila says, “Generally, formulators will be required to avoid reactive materials such as EP additives. Formulators must recognize that metals ‘wet’ differently, so all additives will not be equally effective on every metal alloy.”
Tang’s key performance parameters are listed in Table 2. He says, “With the increased number of additives being introduced into a single formulation to address the multifaceted performance needs, the formulation will naturally become more complex and costly. Stabilizing the formulation also can be more challenging.”
 Table 2. Key performance parameters that formulators need to consider in preparing metalworking fluids for multi-metal alloy applications are shown. Table courtesy of Colonial Chemical.
Table 2. Key performance parameters that formulators need to consider in preparing metalworking fluids for multi-metal alloy applications are shown. Table courtesy of Colonial Chemical.
Three criteria that Eldick points out are important are multifunctionality, superior lubricity on soft and hard alloys and sustainability. He says, “An additive that can provide two or more functions such as emulsification, lubrication and corrosion protection is very beneficial in reducing cost and formulation complexity. Additives that can contribute lubricity to soft (non-ferrous) and hard (ferrous) alloys are available and are mainly self-emulsifying. Formulators do have the option to select additives that are only effective on soft alloys as long as they include other lubricity agents that will be effective on hard alloys to complement their performance.”
Eldick considers sustainability to involve the use of additives derived from renewable resources. He says, “Sustainable manufacturing adds value to materials, components and products while maintaining the availability of natural resources and environmental quality for future generations.”
Cole indicates that the best practice for testing a multi-metal alloy fluid is to utilize extreme conditions to make sure performance is effective on non-ferrous and ferrous metal alloys. Extreme conditions can include increasing water hardness, heat and low dilution rates.
She says, “Testing lubricity performance on a variety of fluids is essential, but coming up with a standard test method and a control test fluid can be challenging. Foaming caused by the addition of corrosion inhibitors may be the most difficult side effect to overcome. Finding the right defoamer may work, but sometimes reformulating the emulsification package may be a better option. Hard water stability testing also should be done because hard water soaps can potentially clog up the lines. In some cases, chemistries such as certain phosphate esters may cause hard water issues.”
Additive types
Additive types that can be effective in fluids used to machine multi-metal alloys and those that are not effective are discussed to assist formulators with choosing the best possible options. Yoder says, “Phosphate esters not only provide good lubrication performance on both metal types but also inhibit aluminum staining. Polymeric esters also are good lubricants for both metal types.”
Two types of additives that can negatively impact multi-metal alloy applications are EP agents and primary amines. Yoder explains, “Traditional sulfurized fats and esters, along with chlorinated paraffins, will do well on ferrous metals but offer no EP benefit for non-ferrous alloys. Additionally, some sulfurized fats and esters could stain some aluminum alloys. Also, some primary amines or other pH boosters will often stain most aluminum alloys.”
Lemke says, “Phosphate esters are some of the most versatile additives for both ferrous and non-ferrous metallurgies.
Woolliscroft adds, “The most common additives that are effective on both alloy types are neutral fatty materials such as complex fatty esters. In markets where chlorinated paraffins are permitted to be used, they work well in combination with esters.”
Harwood claims that low pH borate esters are useful buffering agents and help prevent staining to aluminum alloys while protecting ferrous materials. He says, “Aluminum/yellow metal inhibitors are effective at low treat rates to improve stain performance. Chemistries typically employed are phosphate esters and silicates for aluminum stain prevention and triazoles/thiadiazoles for yellow metals.”
Kumar feels that some conventional additives used are not the best options for formulators. He says, “All conventional alkanolamides, sodium petroleum sulfonates and alcohol ethoxylates are not useful for future formulations except to bring down the cost.”
Eldick’s list of beneficial and detrimental additives for ferrous and non-ferrous alloy is in Table 3 with the focus on semisynthetic fluids. He says, “On the beneficial side, lubricity additives are the most impactful based on cost and performance, and low foam, nonionic emulsifiers are recommended for use due to exhibiting low-foam characteristics.”
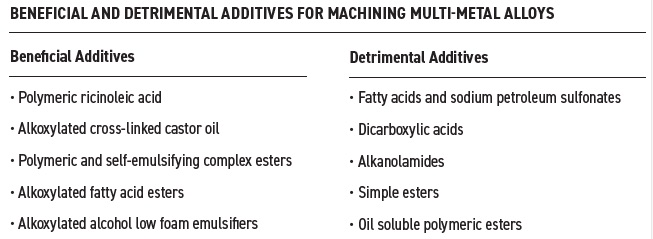 Table 3. A list of beneficial and detrimental additives to use in metalworking fluids for machining multi-metal alloys is shown. Table courtesy of Advanced Chemical Concepts.
Table 3. A list of beneficial and detrimental additives to use in metalworking fluids for machining multi-metal alloys is shown. Table courtesy of Advanced Chemical Concepts.
Polymerized ricinoleic acid and alkoxylated cross-linked castor oil exhibit good multi-metal lubricity and can provide low hydrophilic-lipophilic balance (HLB) and high HLB emulsification properties, respectively. Several different ester types (alkoxylated fatty acid, polymeric and self-emulsifying complex vegetable esters) also are beneficial along with alkoxylated alcohols from vegetable sources for use as low-foam emulsifiers.
These lubricity additives will all work well in semisynthetic fluids, according to Eldick. The polymeric esters should have residual acid so they can easily be emulsified when neutralized with amines.
The common factor among most of the detrimental additives is the formation of aluminum soaps. Eldick says, “The anionic-based additives (fatty acids, sodium petroleum sulfonates and dicarboxylic acids) react with aluminum to form aluminum soaps that are water insoluble and can cause buildup on machines and parts potentially leading to clogged filters.”
Alkanolamides are generally very foamy, simple esters are susceptible to hydrolysis, and oil soluble polymeric esters can cause instability particularly in semisynthetic fluids, according to Eldick.
STLE member Min Chen, global business manager, metalworking fluids for ANGUS Chemical in Buffalo Grove, Ill., indicates that phosphate ester-based chemistries are widely used for aluminum stain inhibition and as EP additives. Triazole-based chemistries provide good protection for copper alloys. In selecting amines for pH neutralization and reserve alkalinity, 3-amino-4-octanol (3A4O), and aminobutanol/aminoethyl propanediol (AB/AEPD) will protect ferrous metals and will also help prevent aluminum and copper staining/leaching while maintaining pH for a longer time due to their low volatility and hindered molecular structures, i.e., primary amine off of a tertiary carbon.”
Figure 4 shows the effect of 3A4O at a 1% treat rate with six other amines when tested on four commonly used aluminum alloys for stain inhibition. 3A4O shows a similar benefit in protecting ferrous alloys when used at a 2% treat rate with five other amines in the cast iron chip test study shown in Figure 5.
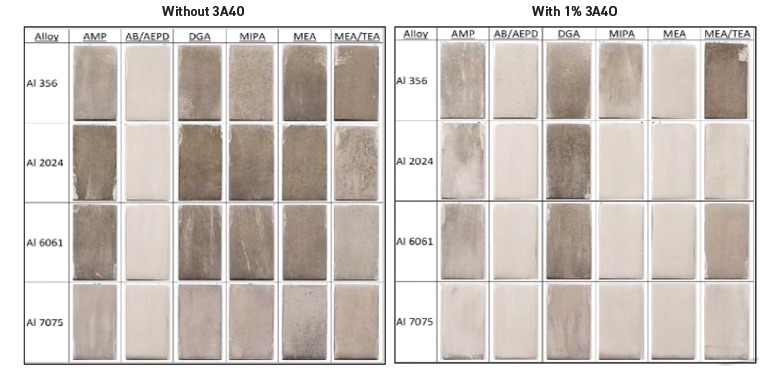 Figure 4. The amine 3A4O shows a benefit in protecting four aluminum alloys from staining when used at a 1% treat rate with six other amines, as shown in the image on the right. Without 3A4O, extensive staining is shown in the image on the left. Figure courtesy of ANGUS Chemical.
Figure 4. The amine 3A4O shows a benefit in protecting four aluminum alloys from staining when used at a 1% treat rate with six other amines, as shown in the image on the right. Without 3A4O, extensive staining is shown in the image on the left. Figure courtesy of ANGUS Chemical.
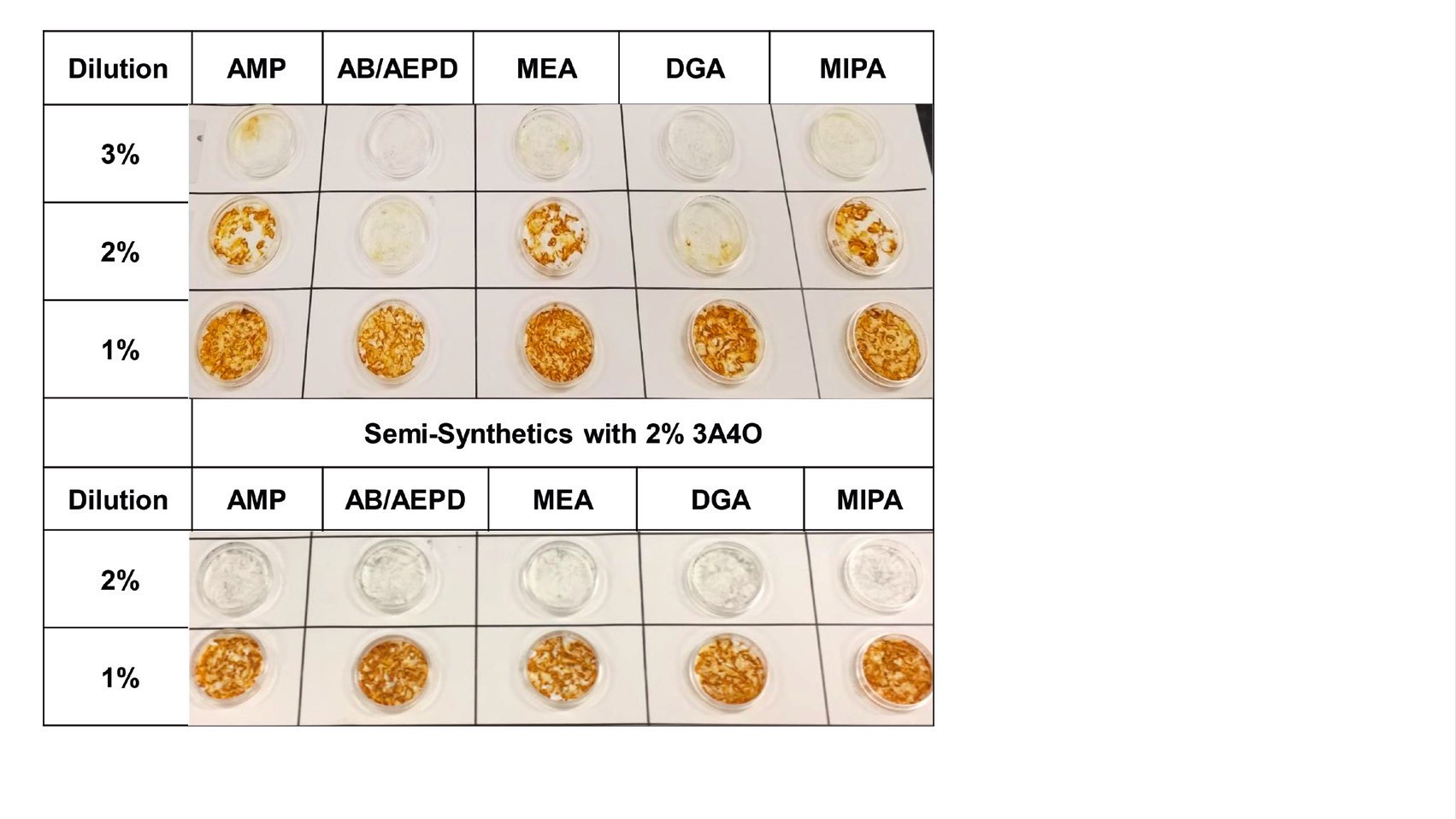
Figure 5. Cast iron chip testing shows that 3A4O has a similar benefit in protection ferrous alloys from corrosion. Images on the bottom show the benefit of 3A4O with five other amines. Without 3A4O, the top images reveal more corrosion on the filter papers. Figure courtesy of ANGUS Chemical.
Cancila believes phosphate esters, esters, hydrocarbon-based fluids and carboxylate chemistry can provide benefits as multi-metal additives. He says, “Phosphate esters generally are multi-functional additives, supplying lubricity, wetting and corrosion inhibiting properties to various metal surfaces. Esters and hydrocarbon-based fluids can offer additional lubricity. Carboxylate chemistry effectively contributes to corrosion inhibition of ferrous and non-ferrous metal surfaces.”
Additives that are ineffective particularly on non-ferrous metals include reactive, EP agents such as chlorinated paraffin, active sulfurized additives and even some phosphorus additives, according to Cancila.
Tang lists phosphate esters and polymeric polyol-based complex esters as additives that can provide corrosion protection, boundary lubrication and EP wear protection on ferrous and non-ferrous alloys.
Overcoming problems using specific additives
One of the most challenging aspects in developing a metalworking fluid that can be used on multi-metal alloys is how to figure out which amines to utilize in a specific formulation. Amines provide necessary reserve alkalinity but can stain aluminum alloys if present at high concentrations.
Stapels says, “The choice of the acid-functional component can significantly improve the final performance of the fluid. Amine salts and especially amine carboxylates can be effective, but there is room for improvement. Ether carboxylic acids have been found to reduce the negative impact of amines staining aluminum alloys.”
Emulsions prepared with an ether carboxylic acid that has less than five ethylene oxide units, and a carbon chain length less than C16, diluted to 5% in deionized water, were evaluated with five amines. The pH of the fluids were adjusted to 9.4 and freshly abraded aluminum plates immersed in the fluids for 24 hours at 40 C. As shown in Figure 6a, a significant reduction in staining was seen for three commonly used aluminum alloys.
Stapels says, “Significantly more staining is seen in Figure 6b on the three aluminum alloys when the same study is conducted with an ether carboxylic acid containing greater than five ethylene units, and a carbon chain length greater than C16. The conclusion from the two studies is the combination of the proper ether carboxylic acid with these amines is decisive in minimizing aluminum staining.”
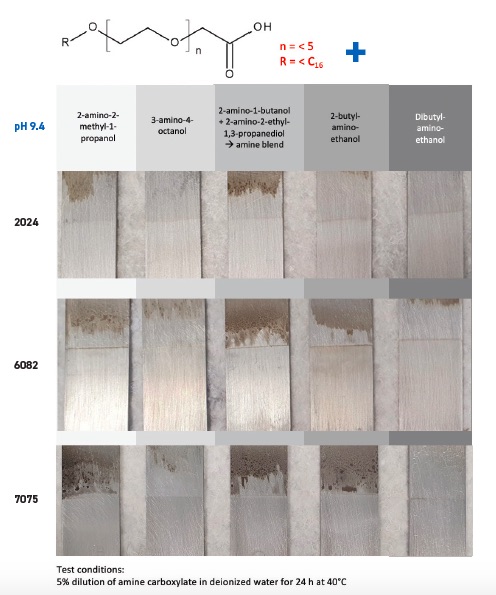 Figure 6a. An ether carboxylic acid with a specific composition can reduce staining in three aluminum alloys when used with five amines. Figure courtesy of Kao Chemicals.
Figure 6a. An ether carboxylic acid with a specific composition can reduce staining in three aluminum alloys when used with five amines. Figure courtesy of Kao Chemicals.

Figure 6b. Evaluation of the same three aluminum alloys, using fluids with the same five amines, but an ether carboxylic acid with a different composition, generates significantly more staining. Figure courtesy of Kao Chemicals.
Eldick believes that single metal specific additives can be blended together to produce a metalworking fluid that is fit for machining multi-metal alloys. He says, “In formulating a multi-metal fluid, it is always preferable to select additives that are fit for use on ferrous and non-ferrous metals. However, if that is not possible, then specific additives fit for one type of metal can be incorporated in a multi-metal formulation. Some caution is required in order to achieve the intended results.”
To illustrate how this formulation approach will work, Eldick provides a low oil semisynthetic fluid that is designed to machine ferrous metals, non-ferrous metals and hard alloys in Table 4. Included in the formulation is a description of the function of each of the additives.
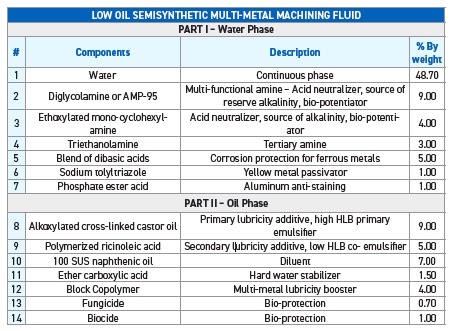 Table 4. A low oil semisynthetic fluid formulation designed to machine ferrous metals, non-ferrous metals and hard alloys is provided with each specific component, the component’s function and treat rate. Table courtesy of Advanced Chemical Concepts.
Table 4. A low oil semisynthetic fluid formulation designed to machine ferrous metals, non-ferrous metals and hard alloys is provided with each specific component, the component’s function and treat rate. Table courtesy of Advanced Chemical Concepts.
Yoder proposes two approaches for overcoming problems that may occur in developing a multi-metal fluid. He says, “Doing a careful design of experiment to study the additive interactions that determine optimum levels is something a formulator can do. Another option is to design the fluid to run at different concentrations for each metal to reduce the detrimental effect of a specific additive.”
Williams says, “A formulator needs to reach a critical balance amongst the additive choices as not all are equally compatible.”
Corrosion inhibitors
Corrosion inhibitors are one of the additives that are at the center of the challenge in formulating fluids that will be effective on ferrous alloys and non-ferrous alloys. The tradeoff between needing a high level of alkalinity to ensure ferrous alloys are protected against corrosion, but making sure this degree of alkalinity does not cause staining of aluminum alloys makes it very difficult for formulators to find a middle ground.
The contributors to this article offer a variety of potential solutions to a complex issue.
Chen says, “Amines such as 3A4O and AB/AEPD when used in combination will provide good corrosion inhibition without staining aluminum or other non-ferrous metals and excellent emulsion stability.”
Harwood says, “Lower pH borate esters are useful buffering agents and help prevent staining to aluminum alloys while protecting ferrous materials. There are a variety of amines that have very good multifunctional properties, which are used where cost is not a factor to prevent their use.”
Kumar says, “Dicarboxylic and tricarboxylic acids with less aggressive amines/alkanolamines supported by a good passivator provide formulators with a good strategy for developing a multi-metal fluid.”
Williams says, “The best option here is to use phosphate esters, carboxylic acid and tertiary amine combinations.”
Yoder says, “Traditional amine carboxylate or amine borate chemistries are effective in this area as they themselves do not stain aluminum in typical metalworking operating pH ranges. Additionally, there are many phosphate esters that can be used at low treat rates to help with aluminum stain inhibition. If all else fails, silicates can be considered as well.”
Eldick feels that a combination of amine neutralized dicarboxylic acids, amine neutralized phosphate esters and sodium tolyltriazole are used to strike a balance between ensuring ferrous alloys do not rust and aluminum alloys do not stain.
Cancila agrees and also proposes that imidazolines be considered as a potential corrosion inhibitor that can be used in multi-metal alloys.
Formulator perspective
Representatives from two metalworking fluid formulators were contacted to get their perspective on the challenges in developing fluids for multi-metal alloy applications. STLE member Bridget Dubbert, technical director at Engineered Lubricants in Maryland Heights, Mo., indicates that finding additives that work well on all metal alloys with one formulation is quite a challenge; finding additives that work well for a couple of alloys is doable. She says, “For example, different aluminum alloys stain/etch from different causes (pH, yellow metal content, etc.), which makes it difficult to find a one-size-fits-all inhibitor when needed for every aluminum alloy. Of the inhibitors available, all have different alloys where they are most effective in inhibiting stain formation. Ensuring proper ferrous corrosion protection without compromising aluminum corrosion can present another multi-metal challenge.”
STLE member Kevin Saunderson, senior technologist at BP Lubricants USA in Naperville, Ill., considers multi-metal compatibility to be one of the many challenges formulators face when selecting additives in developing products. He says, “It is important to understand both the benefits and potential drawbacks of each additive selected for a formulation. Experience has shown that certain amines, while very cost effective, can be extremely aggressive to non-ferrous metals, specifically aluminum alloys. Inhibitors designed to protect or improve compatibility with aluminum may lead to other concerns such as foam or biological issues. Cost also is a consideration as evaluation of newer additive technologies that are designed to provide formulating flexibility typically carry a higher price tag.”
Saunderson feels that the existing additive technology allows for significant flexibility to enable formulators to develop high-performance fluids that meet the wide variety of end-user application requirements. He says, “Challenges still remain, much of which is dependent on the type of alloy being machined as well as the individual system parameters such as speeds and feeds, sump size, water quality, etc. To meet these challenges, the formulator needs to understand the machining characteristics of these alloys to ensure consistent performance and long sump life. For aluminum machining, foam must be controlled and excessive aluminum soap buildup must be avoided because it may stabilize foam in the metalworking fluid system. Current and future additives that help the formulator address these challenges will be very well received.”
Dubbert reveals that there are quite a few current additives for multi-metal applications that work very well. She says, “Additive suppliers have successfully developed products with fewer ‘side effects’ such as extreme foam, incompatibility, bug food, etc. They also recognize additive combinations are often necessary; in turn, they are developing options that are synergistic without causing detrimental side effects when mixed with other additives.”
Saunderson indicates that increasingly restrictive global regulations are limiting additive options that formulators can use in multi-metal alloy fluids. He says, “Of particular importance to multi-metal fluids is the uncertainty surrounding future regulation of phosphorous compounds. This may require significant reformulation of existing product technology to meet the same performance standards. Phosphorus compounds have proven to be highly effective as a multi-functional additive used by formulators to meet today’s more challenging performance requirements despite some drawbacks.”
Regulatory restrictions on boron compounds, particularly in the EU, have created challenges in preparing globally compliant formulations. Saunderson says, “Several global end-users have added boric acid to their restricted substance list.”
As is well known in the metalworking fluid industry, the number of registered biocides in the U.S. and the EU has declined steadily over the past two decades. Saunderson says, “The number shrinks further when looking for biocides that are suitable for in-concentrate use. Concern about using formaldehyde-releasing agents has further limited the choices. The result is that formulators are now developing multi-metal fluids without biocides that can cause performance problems because they exhibit higher levels of alkalinity.”
Dubbert cites the challenge of dealing with the Globally Harmonized System (GHS) where labeling is a big concern for end-users. She says, “Pictograms have probably been our biggest challenge because customers will select a product solely based on them. A workhorse additive, that has been useful in multi-metal applications, now limited due to regulatory scrutiny, is medium chain chlorinated paraffins (MCCPs). This substance class has been under evaluation by EPA in the U.S., and designated as a Substance of Very High Concern (SVHC) by the EU, which will end the use of MCCPs in the EU and may eventually carry over to the U.S.”
Dubbert believes that suppliers furnishing stain test data on multiple metal alloys is very beneficial. She says, “When suppliers provide data showing how one of their products inhibits stain or does not stain certain alloys, but our internal testing does not show the same result, we can troubleshoot with the supplier rather than dismissing the additive as a failure.”
Dubbert indicates that stain testing can correlate well with field results, but other performance testing, while useful, is not always a reliable predictor of field results.
Among the testing that Saunderson recommends is bi-metallic corrosion, bioresistance (ASTM E2275) and tapping torque or a similar test method and foam testing. He says, “Testing should be done by suppliers using a finished formula matrix to account for interactions among functional groups. Independent evaluation of additives provides little value. Bioresistance testing also is useful because it can provide the opportunity to monitor for changes in corrosion protection as the fluid ages.”
Both Dubbert and Saunderson have encountered difficulties in sourcing raw materials. Dubbert says, “We have not had more or fewer issues with multi-metal additives compared to other additives, although our stocking levels have significantly increased to buffer supply chain issues.”
Saunderson says, “To address some of these shortages and improve overall security of supply, formulators have been busy identifying and qualifying alternate sources when available. The ability to act quickly and pivot when necessary has been key to keeping end-users up and running.”
Future challenges
The move toward sustainability will have an impact on the types of additives that will be available to formulators for use in multi-metal alloys. Harwood says, “Environmental impact from ‘cradle to grave’ will become more important leading to the selection of chemistries that are recyclable or biodegradable after use. This leads to asking a few pertinent questions. Will current chemistries be available in the near future because of legislation or public pressure on use or manufacture? How will policies among metalworking fluid manufacturers and their customers adapt as environmental, social and corporate governance (ESG) and carbon footprint impact what is used/accepted? One potential answer is the use of local suppliers to reduce carbon footprint and prevent supply disruption will increase.”
Williams indicates that water-based metalworking fluid technology will continue to evolve in response to ever changing health and safety regulations. Kumar challenges metalworking fluid formulators to innovate as the only path for achieving future success. He adds, “Identification of new combinations of available chemistries is needed to overcome future issues with machining multi-metal alloys. Formulators need to identify-try-confirm.”
STLE member Austin Smith, regional account manager for Clariant in Mount Holly, N.C., stresses the current and future need for identifying alternative options for metalworking fluid formulations. He says, “While it is ideal, and many times desired, to find one or limited products to cover multiple properties and end applications, it is important not to lose sight of having alternative options to supplement in a formulation if and when needed. As technologies continue to advance, multi-property additives also will evolve to check off more and more of the overall performance properties needed. Supply chain considerations must be kept in mind, and formulators must position themselves to be able to adjust their products quickly to meet the end-user’s needs.”
Yoder feels that the demand by end-users for longer lasting fluids will place additional stress on formulators. He says, “Shelf life and emulsion stability requirements placed on multi-metal fluids that are more commonly used in high-pressure machines increases the need for new additives that are low foaming, thermally and hydrolytically stable and soluble in many kinds of base stocks to meet these demands. Further compounding these issues are regulatory actions (such as the upcoming EU SVHC list) that will force formulators to be more cognizant of the additives they choose, and most likely lead to the elimination of some altogether, as end-users want fewer hazardous materials in their facilities.”
Cancila warns that finding new additives that will be acceptable worldwide is becoming more challenging. He says, “The current global network of laws, regulations and registrations is a significant hurdle for additive development making it more difficult to introduce new additives into the marketplace. Metalworking fluid manufacturers want to offer formulations that can be supplied worldwide, but additive availability can be limited due to regional restrictions.”
Potnis says, “Original equipment manufacturers (OEMs) and formulators are increasingly focused on utilizing chemistries with a more environmentally responsible profile to improve the overall product lifecycle of their products. One example is the availability of amines that are multifunctional, readily biodegradable and exhibit low volatility. These components are able to impart high-efficiency neutralization, multi-metal compatibility and extend fluid life.”
Stapels points to three key issues that fluid suppliers will be seeking in preparing multi-metal fluids in the future. He says, “They are security of supply, environmentally friendly additives that are not GHS hazards and multifunctionality.”
Eldick believes that new developments in machining equipment, metallurgy and the move to sustainable chemistries will be some of the future challenges that formulators will need to face. He says, “Suppliers of metalworking additives and finished fluids are constantly adapting to new needs and trends caused by advances in these areas. New developments in CNC machines will lead to faster machining times and smaller sumps where higher fluid flow and pressure will be present. An ever-increasing number of alloys of all metal types (ferrous and non-ferrous) will make it even more challenging to develop formulations that are functional and effective. Sustainable metalworking fluids can be achieved through formulation redesign, use of biobased components, in-line monitoring of bacteria growth and recycling. Once achieved, this will lead to improved cost performance, reduced health and safety risks and reduced environmental emissions.”
As end-users continue to demand fewer metalworking fluids to operate on a wider range of metal alloys, formulators are facing and overcoming this challenge by utilizing what is available in the toolbox. Restrictions on what is available will continue, but enough additive types are available for formulators to devise unique combinations that will meet each particular application.
REFERENCE
1.
Denton, J. (2018), “Metallurgy for the nonmetallurgist with an introduction to surface finish measurement,” in Byers, J. (ed.),
Metalworking Fluids, Third Edition, CRC Press: Boca Raton, Fla., pp. 19-50.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. You can reach him at neilcanter@comcast.net.