KEY CONCEPTS
•
Environmental barrier coatings have been used over the past two decades to protect the hottest sections of jet engines.
•
Environmental barrier coatings can crack at temperatures above 1,200 C.
•
A new duplex bond coating, that involves modifying the existing environmental barrier coating through the use of hafnium dioxide, displays superior thermal stability up to 1,300 C.
Steps continue to be taken to improve the efficiency of gas turbine engines operating in jet aircraft. The challenge in operating a jet engine is combustion temperatures now are in the 1,300-1,500 C range. Higher temperatures are required for jet engine operation because this improves efficiency.
For more than 50 years, lubricants used in the operation of jet engines have been based on polyol ester, synthetic base stocks. The key specification was developed by the U.S. military and still is in use today. In a previous TLT article,
1 a discussion was held about the future of jet lubrication. New technologies under consideration include the use of geared turbo fans that involve the installation of a reduction gearbox inside the engine and replacing the engine with an electric motor. Due to the obstacles for completely electrifying jet engines, hybrid systems are under development, but a lengthy period of time will be required to commercialize such a concept.
The high temperatures encountered in a jet engine also make it difficult to find the right type of materials that can be used in the engine to withstand this extreme environment. Dr. Jeroen Deijkers, a postdoctoral research associate at the University of Virginia in Charlottesville, Va., says, “Over the past 20 years, the main material used in a jet engine is a metallic nickel, cobalt superalloy that is grown as a single crystal. The problem with this metal alloy is that creep deformation and loss of mechanical properties are found at the hotter operating temperatures found in today’s newest jet engines. In an effort to produce fewer emissions and to achieve fuel savings, jet engines are running under leaner conditions, with more oxygen used, when more power is required. This increases the maximum combustion temperature.”
Deijkers continues, “Since the 1990s, the solution to dealing with the temperatures in the hottest sections of jet engines has been to replace the metal alloys with ceramics such as silicon carbide composites. But the problem is that silicon carbide is pretty brittle and only will remain stable for a few thousand hours of flight time under the high jet engine operating temperatures. The carbon present in silicon carbide is removed through reaction with oxygen to form carbon monoxide. At the same time, the silicon forms silicon dioxide that reacts with water vapor to produce gaseous silicon hydroxide, which dissipates from the engine.”
The problem faced in using silicon carbide is to minimize oxidation by reducing exposure of the ceramic to the reactive gas flow coming from the jet engine. Over the past two decades, environmental barrier coating (EBC) systems were developed to protect the silicon carbide.
Deijkers says, “An EBC system currently consists of a two-layer coating where the outer layer reduces the ability of the oxygen and water vapor to reach the silicon carbide and the inner coating is prepared from silicon, which can be oxidized to form a silicon dioxide layer that protects the silicon carbide. The coatings also should exhibit coefficient of thermal expansion values that are close to the silicon carbide. At present, the material of choice for the outer coating is ytterbium disilicate.”
The current EBC has two limitations according to Deijkers. He says, “The presence of high pressure water being generated under the high speed conditions of combustion puts stress on the EBC leading to the formation of silicon hydroxide that can cause the coating to crack. At temperatures in excess of 1,200 C, the silicon dioxide formed during oxidation is a beta cristobalite phase that is more vulnerable to high tensile stress under the jet engine operating conditions leading to cracking and failure of the coating.”
A new approach has now been developed to produce an EBC that is less vulnerable to decomposition.
Hafnon
Deijkers and his colleagues including Haydn Wadley, Edgar Starke professor of materials science and engineering at the University of Virginia, modified the currently used EBC by adding a thin layer of hafnium oxide between the ytterbium disilicate and silicon layers to form a duplex bond coating. He says, “We had a decision about how to reduce the failure of the existing EBC. One strategy involves thickening the outer coating to reduce the ability of oxygen to penetrate to the silicon carbide. This idea would add weight to the coating, which is not desirable as it would reduce efficiency and increase cost. Instead, we decided to develop a new approach in which hafnium silicate, also known as hafnon, is generated as the coating is in use.”
The researchers selected hafnon because of its exceptional match in thermal expansion up to 1,500 C. Deijkers says, “Hafnium dioxide is able to maintain its monoclinic crystal structure up to that temperature with no evidence of volume change or phase deformation, without fear of crack formation in the hafnium oxide or hafnon layers.”
In preparing the coating, the researchers used a directed vapor deposition process to prepare the silicon-coated silicon carbide. A coaxial-plasma assisted, direct vapor deposition method was used to apply hafnium dioxide to the silicon followed by application of ytterbium disilicate. The key to the process is that as the silicon coating layer is oxidized, silicon dioxide reacts with hafnium dioxide to produce hafnon.
The researchers evaluated the duplex bond coating using a steam cycling approach where the coating is heated up to 1,000 cycles between 110 and 1,316 C. Figure 2 shows a photograph of a surface coating with the hafnon-produced EBC after 1,000 cycles. This image shows there is no evidence of decomposition within the hafnium coated surface. Outside of that surface, delamination of the silicon, silicon dioxide interface and dark regions where surface cracking occurred were observed by the researchers.
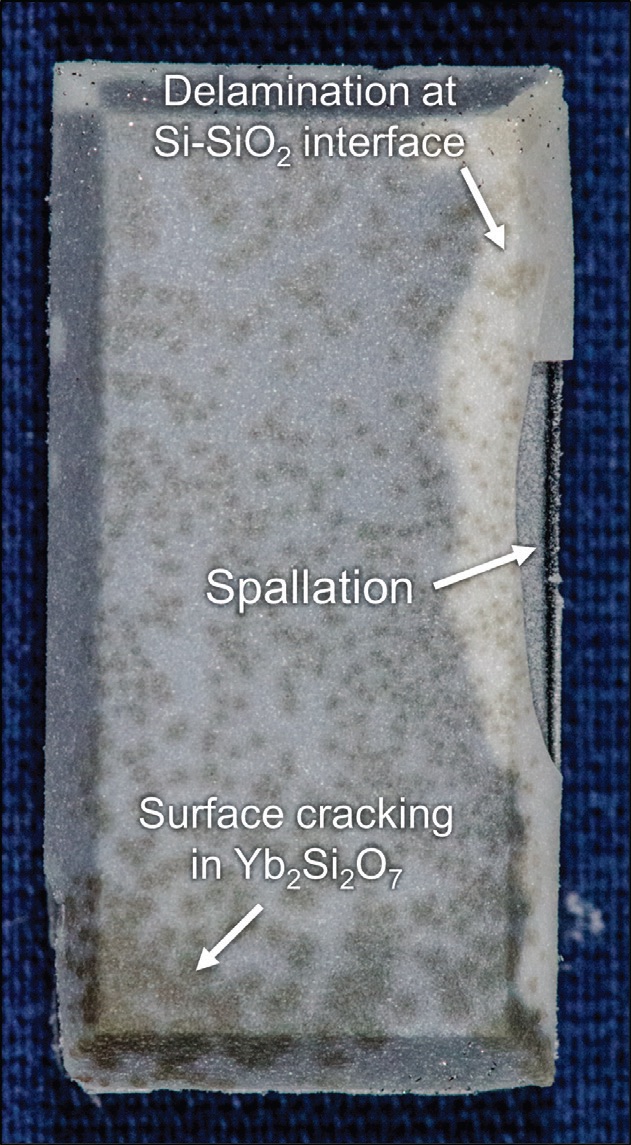 Figure 2. Production of hafnon in the environmental barrier coating produces no evidence of decomposition after 1,000 cycles between 110 and 1,316 C as shown in the figure. Figure courtesy of the University of Virginia.
Figure 2. Production of hafnon in the environmental barrier coating produces no evidence of decomposition after 1,000 cycles between 110 and 1,316 C as shown in the figure. Figure courtesy of the University of Virginia.
Future work will include trying to commercialize this duplex bond coating, produce coatings with better properties and working with different materials. Deijkers says, “We would like to develop coatings that exhibit lower thermal conductivity, generate lower amounts of thermal radiation and also are mechanically stable.”
Additional information can be found in a recent article
2 or by contacting Deijkers at
jeroen@virginia.edu and Wadley at
haydn@virginia.edu.
REFERENCES
1.
McCoy, B., (2021), “The future of jet engine lubrication,” TLT,
77 (10), pp. 40-45. Available
here.
2.
Deijkers, J. and Wadley, H. (2021), “A duplex bond coat approach to environmental barrier coating systems,”
Acta Materialia, 217, 117167.