KEY CONCEPTS
•
It has been 80 years since the first patents were published for ZDDP as a lubricants additive.
•
ZDDP’s popularity with additives suppliers and oil companies is based on a combination of effectiveness in an application, relatively simple manufacturing process and relatively low cost.
•
The enormous datasets compiled by the additives suppliers and oil companies during years of bench, rig and engine testing anchors current and future engine oil developments, particularly where backward compatibility is required.
2021 marked the 80th anniversary of the publication of the first patents mentioning the use of zinc dialkydithiophosphate (ZDDP) as an additive in engine oils. Over the years, many molecules that could replace ZDDP have never made the market. This, despite their having compelling technical or health, safety and environment (HSE) claims, such as better extreme-pressure/antiwear (AW) performance, lower environmental impact, less or no metals, better human health profile, etc. Most are ignored or politely rejected. Why?
This article explains the background to the additives suppliers’ and lubricants marketers’ heavy investment in ZDDP over the years and why—with mass electrification around the corner— there is little incentive to change.
Many who have worked in the lubricants industry over the last 20 years have had regular approaches from sales staff, start-ups and/or university groups promoting a proposed replacement molecule for ZDDP and seeking interest or funding. For those who attend conferences, it can seem like there are one or two poster presentations every time!
The pitches to funders often focus on the value to investors if these molecules replace ZDDP in all automotive crankcase lubricants globally. Few are successful, and none have made the whole 10 yards. Why?
Steve Haffner, an independent consultant in the lubricants industry, says, “ZDDP affects formulations in many ways. It is a complex component that is activated by higher temperatures and, although it is best known as an AW component, it’s a powerful antioxidant, helps prevent corrosion and has been shown to have a positive impact preventing low-speed pre-ignition (LSPI). It is field-proven over decades and is one of the most cost-effective components in additive company tool kits.”
STLE member Marc Ingram of UK-based consultancy Ingram Tribology offers a tribologist’s appreciation of ZDDP: “To my knowledge, an AW additive that is on par with ZDDP has not yet been found. ZDDP has some performance attributes that surpass other AW technologies—it can form a thick tribofilm very quickly, it has very good wear protection, and it can perform these functions on many different types of steels and other materials
(see A Test of Its Own: MTM-SLIM).”
A test of its own: MTM-SLIM
The deeply embedded nature of ZDDP films in tribology was articulated in a light-hearted manner by Marc Ingram. “ZDDPs are so important, they even have their own test.”
As the thickness of AW films is the same order of magnitude as the wavelength of visible light, it was possible to adapt a tribometer by applying the principles of a piece of equipment previously used to measure the thickness of elastohydrodynamic (EHD) films between a ball and glass disc.
1
“The mini-traction machine (MTM) is a ball-on-disc tribometer, which allows a combination of load, speed, slide roll and temperature to be set in a predetermined sequence,” says Matt Smeeth of PCS Instruments. While the origins of the MTM were in the understanding of ZDDP-derived AW films, it can study any tribofilm. “The materials of both the ball and the disc can be changed—ceramics, alloys and many surface coatings have been studied. For example, many diamond-like carbon (DLC) surfaces are sufficiently reflective for study.”
“The origins of the spacer layer imaging (SLIM) attachment come from ball-on-glass disc interferometry equipment that measured film thickness in the EHD regime,” Smeeth continues.
Figure 1 shows a schematic of the rig in film-forming mode.
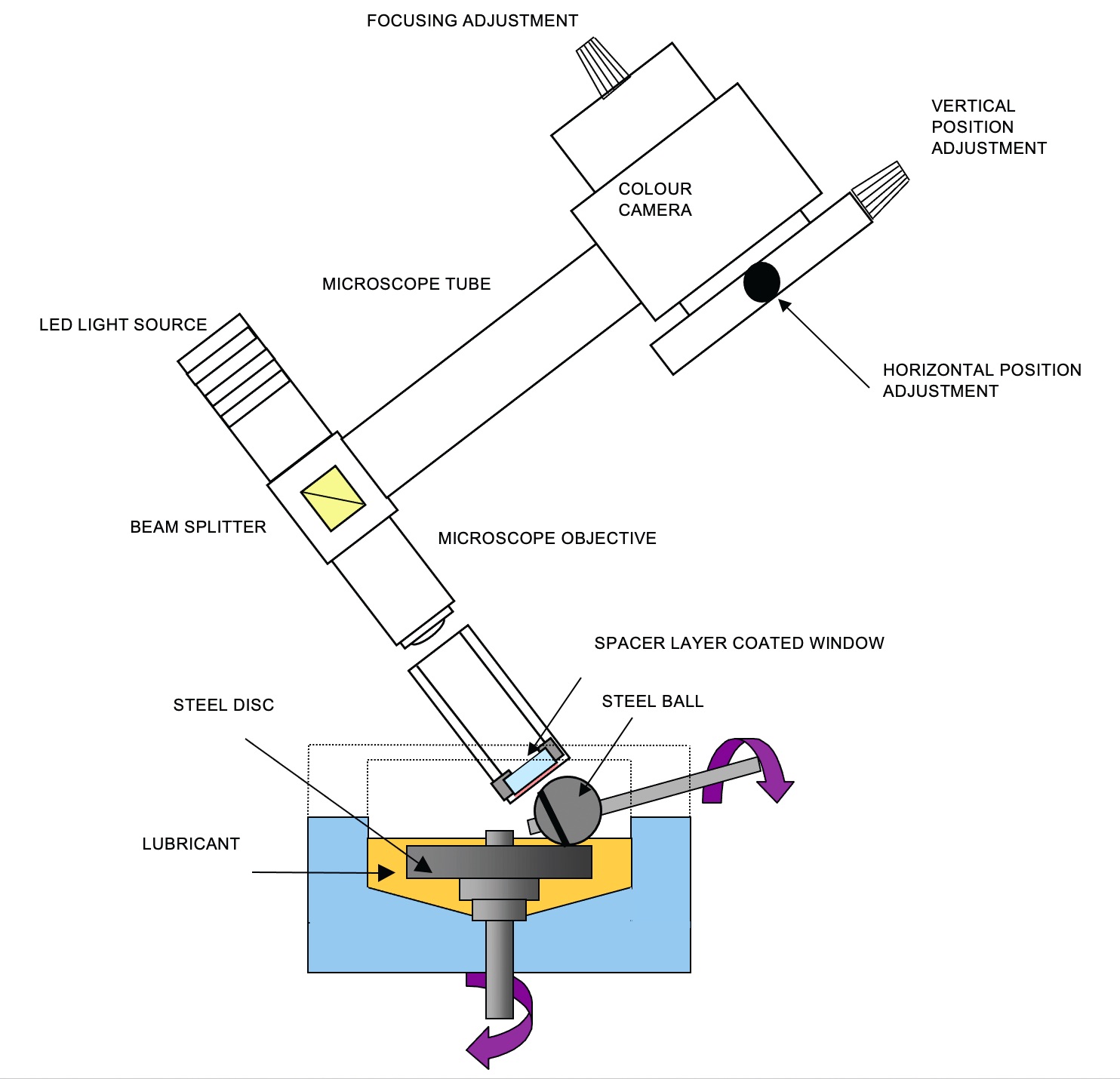 Figure 1. Schematic of the rig in film-forming mode. Figure courtesy of PCS Instruments.
Figure 1. Schematic of the rig in film-forming mode. Figure courtesy of PCS Instruments.
The ball is first loaded against a flat disc, which is immersed in the test oil sample. The ball and the disc are continuously under mixed sliding and rolling conditions, such that an adsorbed tribofilm forms on the surfaces of the contact. At predefined intervals, the rotation is paused, and the ball is automatically reverse loaded against a coated glass window. An interference image of the contact between the ball and the glass window is captured by a high-resolution CCD camera, from which the tribofilm thickness can be measured.
“Tests can typically be carried out in a few hours,” says Smeeth, and at the end of the test, the film on the ball can be analysed chemically and physically.
Figure 2 shows the development of a film on the steel ball.
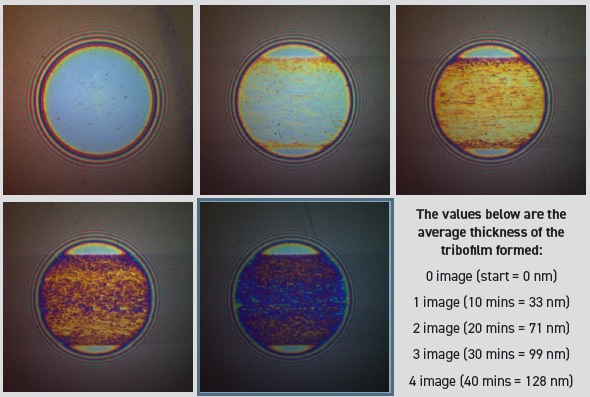 Figure 2. The images show the development of the tribofilm over time. The images were taken at 10 minute intervals. The oil contained a relatively fast acting secondary ZDDP. Figure courtesy of PCS Instruments.
Figure 2. The images show the development of the tribofilm over time. The images were taken at 10 minute intervals. The oil contained a relatively fast acting secondary ZDDP. Figure courtesy of PCS Instruments.
To Afton Chemical’s Ian Bell, the answer is simple: “ZDDPs are better and cheaper than any alternative for the various jobs they perform in a lubricant. If there was anything cheaper, we would be using it.” Bell’s response touches on a significant feature of ZDDP— it is not a single molecule, so there is potential to fine tune activity while still using relatively cheap raw materials in a relatively simple manufacturing process.
Haffner adds some other reasons why ZDDPs are difficult to replace. “The additive companies have significant capital investment in manufacturing facilitates for ZDDP, so there’s very little incentive to spend the millions in R&D or build new facilities to try and replace it, unless there are further mandates to further reduce levels of sulfur, phosphorus or zinc.”
There’s another heavy investment that would be required to displace ZDDPs from automotive crankcase lubricants in their final decade or so of existence, and that’s the cumulative historical investment in rig, engine testing and field trial data, almost all of which are on formulations based on ZDDPs. This covers all internal combustion engines (ICEs): passenger cars, trucks, rail vehicles, agricultural machinery, motorcycles, boats or ships. As the ICE has remained the largest consumer of lubricants for more than 80 years, the status of ZDDP as the go-to AW additive has been assured. To this, the observer should add the other functionality it brings.
In recent years, ZDDP has been referred to as the most successful lubricants additive
2 and the accidental additive,
3 as the publication of AW benefits of ZDDPs post-dated patents on their effectiveness as antioxidants or corrosion preventives. So, a little history of this amazing molecular type is probably in order.
History
An in-depth review of ZDDPs and their importance to lubricants was published in 2004 by STLE Fellow and Life Member professor Hugh Spikes of Imperial College London.
2 A summary of this extensive history is that in 1941, Lubri-Zol (the forerunner of Lubrizol), American Cyanamid and Union Oil Co. each filed patents with claims of corrosion and oxidation inhibition in engine oils. The Lubrizol patent was granted in 1941 and the others in 1944.
The antioxidant activity of ZDDPs is acknowledged, even today, as one of the seminal tomes on lubricants additives—“Lubricant Additives Chemistry and Applications, Third Edition,”
4 includes the ZDDP chapter in the section “Deposit Control Additives: Oxidation Inhibitors,” rather than the following “Film Forming Additives” section.
An early patent containing reference to ZDDP as an AW agent belongs to Exxon, filed in November 1954 and granted in February 1958.
5 By this time, V8 engines with overhead valves were in the market, which suffered from cam and follower wear in the valve train. Successful deployment of ZDDP-containing lubricating oils to address this cemented their use for the next 50-60 years.
Phil Reeve of ADLU Consultancy, based in the UK, says, “Initially ZDDP was used in low doses to provide a level of AW and antioxidancy to mineral-based formulations. Over time, ZDDP became a staple component in almost all formulations providing a highly cost-effective solution for AW, antioxidancy and corrosion inhibition. As new engine tests emerged, formulators explored different aspects of ZDDP, such as treat rate curves and chemical variations in alkyl chain type and length,” all of which based the new formulations on their successful forebears.
Chemical variations
The chemical route to ZDDPs is relatively simple. Highly pyrophoric phosphorus pentasulfide is reacted with a slight excess of an organic alcohol, and this mixture is then treated with zinc oxide. Purification is minimal, with water and excess alcohol being removed by vacuum distillation and excess zinc oxide being removed by filtration.
4 However, a by-product of the first stage is highly toxic hydrogen sulfide (H
2S), which must be disposed of safely. This involves either a further reaction of H
2S with a caustic solution, from which H
2S may be liberated later for use in other chemical processes, or combustion to produce sulfur oxides. However, as these sulfur oxides are very acidic, their release to air is heavily regulated.
The principal variation in chemistry for ZDDPs is the type and length (and their ratio) of the alkyl chains, which are derived from the alcohols. “The hydrocarbon chain can be aryl or alkyl,” Reeve explains. “If an alkyl chain is used, this can be either primary or secondary and have a chain length typically between C
3 and C
8. More recent specifications limiting phosphorus content of engine oils and emphasizing phosphorus retention have tended to favor more secondary alkyl products. The need for greater fuel economy has been supported by limiting the ZDDP level as well.”
Secondary ZDDPs have better extreme-pressure performance and are more thermally labile, so they are often used in factory-fill lubricants where there is a requirement to lay down a film quickly, and the lifetime of the oil is relatively short. Service fill oils—whether supplied to workshops under license to the original equipment manufacturer (OEM) or carrying well-known brand names—will often contain more thermally stable ZDDPs, as those fluids will be in European or Japanese vehicles for up to six times the mileage of the factory-fill lubricant.
Aryl ZDDPs, which also were discovered in the 1940s, have limited use in automotive crankcase lubricants. They are both more thermally stable and less effective antioxidants. However, both features can be an advantage in hydraulics formulations, where there is no external heat source, so less thermal oxidation.
The availability of many relatively cheap organic alcohols could have led to a proliferation of products, but the reality is that a small number of ZDDPs are in regular use. “Whilst the manufacturing cost of ZDDP is relatively cheap, logistically it is not appropriate to have a huge number of ZDDP grades in the portfolio,” says Reeve. Each additive company has its unique chemistry, but these components tend to have limited use. For security of supply, the major Western additives suppliers often have their competitors as secondary suppliers of their large volume ZDDPs.
Versatility
So, even in the 1950s, ZDDPs were recognized for their efficacy as antioxidants, AW additives and corrosion preventives. Synergies were recognized early on, such as with polyricinoleic acid,
5 but it was the drive for fuel economy through lower friction that demonstrated a further aspect of ZDDP’s benefits, and a few problems.
The oil crisis of the 1970s brought a focus on fuel economy and the effect of friction modifiers in the parts of the engine where boundary lubrication was important, such as the valve train and the piston/liner interface. Many organic materials were found to reduce friction in bench tests but failed to show benefits in durability tests, indicating relatively rapid decomposition or deactivation during the lifetime of a lubricant.
Of more interest from a tribological perspective were those that showed friction and wear benefits in full formulations
6 and understanding why some others reduced friction in a rig and were durable but adversely affected by the AW performance of ZDDPs.
A theory emerged of surface competition whereby carboxylic acids and some esters would bind strongly to the surface to reduce friction in a base oil. However, in a full formulation, they would displace or prevent the full formation of an AW film. Industry gradually migrated to either oleyl amides or glycerol monooleate
7 as ashless friction modifiers, where the amide or glycerol ester head group that adsorbs onto the metal surface is less polar, so more labile.
Gareth Moody of Croda Europe Ltd. offers an observation about tribofilms and friction modifiers, where there is a balance between performance and solubility: “Often we see that friction modifiers, which are not fully soluble (cause haze, etc.), work really well at reducing friction. The balance is getting the friction modifier to the surface as efficiently as possible without compromising the stability of the formulated lubricant. This also is true for polymeric friction modifiers.”
During the 1990s, Japanese OEMs were taking an interest in molybdenum-based friction modifiers. This was partly due to concerns about the durability of organic friction modifiers. While some studies focused on molybdenum dithiophosphates, it was soon recognized that there was little performance difference when mixed with ZDDP if molybdenum dithiocarbamates were used.
8, 9, 10 Thus, a theory emerged of ZDDP as a phosphate donor to molybdenum emerged.
11
STLE member Vince Gatto of Vanderbilt Chemicals LLC says, “In engine oils, ZDDP functions by forming various glassy polyphosphate films, which are very effective at reducing wear but not very good at reducing friction. Molybdenum dithiocarbamates function in a way that synergizes with ZDDP by producing more durable friction reducing MoS
2 tribofilms.
10 Replenishment of the MoS
2 tribofims is enhanced by ligand exchange between ZDDP and MoDTC.”
11
More recently, ZDDPs have been shown to reduce the incidence of LSPI,
12 the scourge of small direct-injection gasoline engines. But there’s no happy ending to this part of the story, according to Steve Haffner. “One of the main additive suppliers has reported up to 90% reduction in LSPI events when overtreating some formulations with ZDDP. Unfortunately, chemical limits contained in both American Petroleum Institute (API) and the European Automobile Manufacturers Association (ACEA) specifications do not allow higher phosphorus concentrations in lubricants designed to protect three-way catalysts.”
Limiting variation
The principal drivers for minimizing chemical variation are the cost of engine testing and the codes of practice around component interchange or treat rate modification during testing programs. Bell adds another factor: “Backward compatibility can be very important.” This favors the use of chemistry that succeeded before.
Most passenger car or diesel engine oils have their foundations in industry standard specifications from the API, ACEA, International Lubricant Standards Association (ILSAC) or Japanese Automotive Standards Organization (JASO). However, to minimize the number of fluids in the market, most automotive crankcase lubricants meet two or more of these specifications, plus a few specifications of the market-leading OEMs.
A new industry specification, say an API/ILSAC specification in North America or ACEA specification in Europe, will still have some tests and pass/fail criteria that are the same as its predecessor. “Examining earlier tests with similar test conditions and/or hardware can give strong clues to likely ZDDP responses when faced with a new emerging test,” says Reeve. It’s as important to identify where a formulation could slip from “pass” to “fail” with the new formulation in existing tests, as it is to identify where it might “pass” in a new test. “Historical experience and data can help lubricant formulators funnel down options when working in a similar performance area but is limited in effectiveness when extrapolating to address new or different challenges,” says Bell. If the funneling down process makes it appear likely that the performance requirements can be achieved using existing chemistry, it further strengthens the foundations based on ZDDP.
Preparing for a new specification, the oil marketers will have a list of viscosity grades in their target markets, and blenders will have a list of base oils and their favored viscosity modifier that they would wish to use. In most circumstances, all these factors are known up front and fed into the design of the testing matrix for the oil development program. “These programs are conducted using guidelines within various codes of practice, which the developers sign up for and are audited against,” says Reeve. The guidelines cover the degrees of flexibility the formulators have. These include how many formulation changes can be made during development, which viscosity grades can be read across to other viscosity grades and how much the viscosity modifier treat rate can change, for example. “In general, the program design will aim to run tests to cover the maximum viscosity grade range. So, wear tests are performed at the lowest viscosity of the fluids in the matrix and deposit tests at the highest viscosity modifier treat rate. Here the program manager will create a testing matrix aimed at minimizing test cost and optimizing coverage.” As much of the initial formulation likely is to be based on existing chemistry, the anchor of ZDDP as the “go-to” multifunctional additive is strengthened.
According to Reeve, “Databases have built up around a few key primary, secondary and mixed primary/secondary ZDDPs,” and these data often are generated in separate phases exploring antioxidancy and AW. “Experimental designs for antioxidancy start with bench oxidation tests, which are reasonably cheap and, in some instances, give good directional performance information. This then leads to exploration in engine tests designed to evaluate oxidation performance. Designs for wear performance traditionally have been more difficult. There are several simple tribological tests that can be used, leading on to bench tests such as motored camshaft-type tests. However, the range of different wear scenarios in engines has proved difficult to correlate with laboratory or bench tests. Low-temperature camshaft/tappet wear is very different from high-temperature piston ring/liner wear, for example.”
Bell adds a different dimension: same property, different engine type. “At the moment, I don’t think the industry has the data that allows confidence that we can universally deploy formulation insights across platforms. What is needed for good cam wear performance in, say, the OM 646LA (diesel-powered engine) doesn’t transfer across to cam wear performance in the gasoline-powered engine in the Sequence IVA.” Both engine tests have been part of major qualification sequences for more than a decade.
It is this indistinct correlation that, at the same time, drives the incremental approach to product development and the continuing fundamental studies of many tribology laboratories. If we think of the current set of performance requirements as the surface of a sand dune, the data on different historical formulations provide some very deep soil anchors. However, we have very little effective netting or vegetation across the surface that allows us to read across between the tops of the different anchors, so the surface of the dune is not solid.
Appetite studies
The anchors are strengthened by appetite studies: the assessment of how a particular (new) test responds to different chemistry. “When you design a matrix to investigate test responses, you are looking to run a minimum number of tests to cover a number of identified variables,” explains Reeve. “These could be chemistry or operational variables. ZDDP type or treat rate, dispersant level or effect of friction modifier would be valid formulating variables, while operational variables could be fuel type, test length, oil temperature or feed pressures, among other parameters. These are generally run to develop a new test or understand chemical response via a statistically designed experiment.”
The final hurrah?
One emerging factor that could see the replacement of ZDDPs in some automotive crankcase formulations, according to Bell, is the OEM-specific formulation. With OEMs ramping up, the technical demands on new formulations for factory or service fill and, therefore, being less concerned about whether they work elsewhere, there is the possibility—albeit remote—of a “blank sheet” approach, with historical use giving little or no advantage to each ingredient.
Then there’s the forthcoming electrification of the passenger car fleet. “ZDDPs are not hardwired into thinking on new technology,” says Bell, “but they are definitely still suitable for ICEs and, therefore, hybrids.”
That said, there is still the possibility of ZDDPs offering one more gift to formulators. Bell believes that formulators haven’t fully “mined” the information in their extensive databases. “We have all of this data: chemistry, viscometrics, inlet flow rates, temperatures, etc. If we can characterize this at the engineering level, we can then begin to understand why some formulations work well in certain engines and not in others.”
This probably requires artificial intelligence and is not an academic exercise, as proving the AI models on the enormous data mountains built on ZDDP could well enable their deployment on the development of fluids for the next generation of vehicles.
REFERENCES
1.
Fujita, H., Glovnea, R.P. and Spikes, H.A. (2005), “Study of zinc dialkydithiophosphate antiwear film formation and removal processes, part I: Experimental,”
Tribology Transactions, 48, pp. 558-566. Available
here.
2.
Spikes, H. (2004), “The history and mechanisms of ZDDP,”
Tribology Letters, 17: p. 469. Available
here.
3.
Pedersen, A. (2017), “ZDDP: The accidental additive,” UL Prospector. Available
here.
4.
Rudnick, L.R., ed. (2017),
Lubricant Additives Chemistry and Applications, Third Edition, CRC Press, ISBN: 9781498731720.
5.
U.S. Patent 2824836, granted to Esso Research and Engineering Co., 1958.
6.
Griffiths, D.W. and Smith, D.J. (1985), “The importance of friction modifiers in the formulation of fuel efficient engine oils,” SAE paper 852112. Available
here.
7.
Kenbeek, D., Buenemann, T. and Rieffe, H. (2000), “Review of organic friction modifiers - Contribution to fuel efficiency?,” SAE Technical Paper 2000-01-1792. Available
here.
8.
Unnikrishnan, R., Jain, M.C., Harinarayan, A.K., Mehta, A.K. (February 2002), “Additive-additive interaction: An XPS study of the effect of ZDDP on the AW/EP characteristic of molybdenum based additives,”
Wear, 252 (3-4): pp. 240-249. Available
here.
9.
Bec, S., Tonck, A., Georges, J.M., et al. (2004), Synergistic effects of MoDTC and ZDTP on frictional behaviour of tribofilms at the nanometer scale,
Tribology Letters, 17, pp. 797-809. Available
here.
10.
U.S. Patent 5,744,430, assigned to JXTG Nippon Oil and Energy Corp., 1998.
11.
Kiw, Y.M., Schaeffer, P., Adam, P., Thiebaut, B., Boyerb, C. and Papin, G. (2020), “Ligand exchange processes between molybdenum and zinc additives in lubricants: evidence from NMR (1H, 13C, 31P) and HPLC-MS analysis,”
RSC Adv., 10, 37962.
12.
Ritchie, A., Boese, D. and Young, A. (2016), “Controlling low-speed pre-ignition in modern automotive equipment part 3: Identification of key additive component types and other lubricant composition effects on low-speed pre-ignition,”
SAE Int. J. Engines, 9 (2): pp. 832-840. Available
here.
STLE member Trevor Gauntlett is a freelance writer and consultant on lubricants based in Wirral, UK. You can contact him at trevor@gauntlettconsulting.co.uk.