KEY CONCEPTS
•
An all-solid state battery with an all-silicon anode was prepared.
•
This battery showed good capacity retention when subjected to 500 cycles at room temperature.
•
The use of an all-silicon anode appears to provide better battery operating flexibility while retaining high energy densities.
Improvements in the performance, reliability and safety of lithium-based batteries are ongoing as researchers recognize that the shortcomings of this technology must be addressed before applications, such as electric vehicles, become fully commercialized. One example of this approach has been work conducted to better understand why lithium-metal batteries fail.
In a previous TLT article,
1 a study indicated that lithium-metal batteries fail because a percentage of the lithium metal becomes inactive during each charge/discharge cycle, causing the cell to continuously lose capacity until eventual failure. The researchers determined that the inactive lithium metal forms as a result of being trapped within the solid electrolyte interphase (SEI) that forms as the battery cycles. The SEI contains electrolyte salts such as lithium carbonate and lithium oxide that isolates lithium metal, preventing it from engaging in battery operation.
One of the weaknesses of lithium-ion batteries has been the use of a graphite anode. An attractive alternative is to use silicon as the anode because its energy density is 10 times higher as compared to graphite anodes. But the problem is the poor stability of silicon anodes in lithium-ion batteries.
Ying Shirley Meng, Zable endowed chair professor in energy technologies and professor of nanoengineering and materials science at the University of California San Diego in La Jolla, Calif., says, “The organic liquid electrolytes used in lithium-ion batteries have proven to be incompatible with silicon anodes. Interactions between the liquid electrolytes and lithium-silicon alloy have led to the growth of an unstable SEI that has hindered battery performance leading to poor cycling and shelf life.”
A new approach has now been developed to produce a lithium-ion battery with a stable all-silicon anode.
Sulfide-based solid electrolyte
The researchers decided the best approach to incorporate an all-silicon anode was to use a solid state electrolyte (SSE) in an all-solid state battery. Meng says, “We prepared a battery that contains a 99.9% by weight microsilicon electrode, a sulfide-based solid electrolyte and a cathode prepared from lithium nickel cobalt manganese oxide. In designing this battery, we knew that the cathode was compatible with the solid electrolyte, meaning that battery performance is mainly dependent on how these components interact with the silicon anode.”
During the initial discharge/charge cycle, the SEI is formed initially between the microsilicon anode and the SSE. This was followed by lithiation of silicon and direct ionic and electronic contact between lithium-silicon and microsilicon particles that was found to be highly reversible. This process is shown in Figure 1.
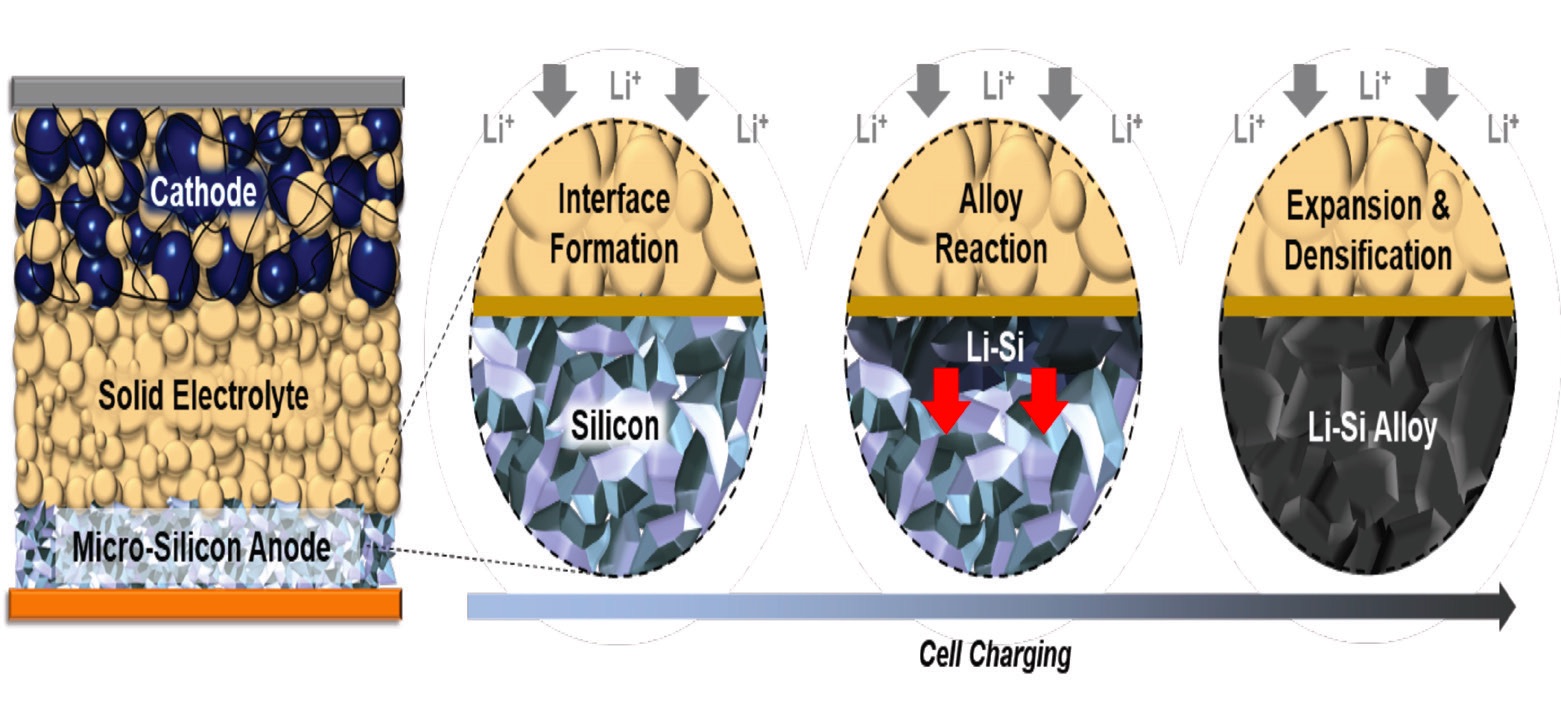 Figure 1. The charging process for an all-solid state battery with a carbon free micro-silicon anode is shown. In the image on the extreme left, the components of the battery are shown. Positive lithium ions are shown moving from the cathode to the anode in the next figure to the right, and a SEI is forming between the solid electrolyte and the micro-silicon anode. The next figure to the right shows that as charging continues, lithium ions in the anode react with the micro-silicon to form lithium-silicon (Li-Si) particles. The figure on the extreme right shows that the charging cycle leads to an expansion and densification process of the micro-silicon particles resulting in the formation of a dense, stable Li-Si alloy electrode. Figure courtesy of the University of California San Diego.
Figure 1. The charging process for an all-solid state battery with a carbon free micro-silicon anode is shown. In the image on the extreme left, the components of the battery are shown. Positive lithium ions are shown moving from the cathode to the anode in the next figure to the right, and a SEI is forming between the solid electrolyte and the micro-silicon anode. The next figure to the right shows that as charging continues, lithium ions in the anode react with the micro-silicon to form lithium-silicon (Li-Si) particles. The figure on the extreme right shows that the charging cycle leads to an expansion and densification process of the micro-silicon particles resulting in the formation of a dense, stable Li-Si alloy electrode. Figure courtesy of the University of California San Diego.
Meng says, “We found that the SEI generated during initial operation of the battery was very thin and stable. More importantly, the SEI did not grow or change upon further cycling providing an indication of the battery’s stability.”
The researchers found that current densities up to 5 milliamperes per square centimeter were achieved at room temperature, and the battery also can operate between -20 C and 80 C. A capacity retention of 80% was achieved when the battery was subjected to 500 cycles at room temperature. This result indicates the potential robustness of a solid-state lithium-ion battery built with an all-silicon anode.
Meng pointed out that although metallic lithium has been the conventional choice for use as an anode in all-solid state batteries, the use of lithium metal also has restricted battery charging rates and forced charging to take place at 60 C or above. Silicon-based anodes appear to provide better operating flexibility, while retaining high energy densities.
To show that the presence of carbon in the anode can lead to inferior performance, the researchers prepared an identical all-solid state battery with the addition of 20% carbon by weight to the silicon anode. They found that the battery containing carbon underwent severe electrolyte decomposition leading to the formation of lithium sulfide.
 One of the weaknesses of lithium-ion batteries has been the use of a graphite anode. An attractive alternative is to use silicon as the anode because its energy density is 10 times higher as compared to graphite anodes.
One of the weaknesses of lithium-ion batteries has been the use of a graphite anode. An attractive alternative is to use silicon as the anode because its energy density is 10 times higher as compared to graphite anodes.
A morphological study on the battery was conducted by preparing cross-section scanning electron microscopy (SEM) images of three microsilicon electrodes. Meng says, “We observed that the anode becomes densified after lithiation with the entire electrode becoming an interconnected lithium-silicon alloy. The electrode does not revert to its original microparticle structure after delithiation but rather converts to discrete, micro-sized particles with voids between them. The phenomenon was highly reversible and is key toward enabling stable charge/discharge of the battery.”
Meng recognizes that improvements are needed in this all-silicon anode battery if this technology is to be commercialized. She says, “The capacity fade we observed is due to the loss of contact between the cathode and the SSE. One challenge with solid-state batteries is the need to optimize stack pressure, which is needed to ensure that battery components such as the SSEs and electrodes remain in good contact with each other over a long operating period.”
Meng indicates that the researchers intend to make bigger cells to better determine how to improve the performance of the battery. She says, “Our work shows that an all-solid state battery with a silicon anode has potential to overcome the limitations of currently used batteries.”
Additional information can be found in a recent article
2 or by contacting Meng at
shirleymeng@ucsd.edu.
REFERENCES
1.
Canter, N. (2019), “Determining how lithium-metal batteries fail,” TLT,
75 (12), pp. 12-13. Available
here.
2.
Tan, D., Chen, Y., Yang, H., Bao, W., Sreenarayanan, B., Doux, J., Li, W., Lu, B., Ham, S., Sayahpour, B., Scharf, J., Wu, E., Deysher, G., Han, H., Jah, H., Joeng, H., Lee, J., Chen, Z. and Meng, Y. (2021), “Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes,”
Science, 373 (6562), pp. 1494-1499.