More on mouthfeel: Imbibing bubbly beverages
Drs. Wilfred T. Tysoe & Nicholas D. Spencer | TLT Cutting Edge December 2021
Carbonation adds another dimension to the mouthfeel of drinks, with effects ranging from the purely physical to the biochemical.

We previously discussed the tribology of red wine,
1 noting that tannins can exert a significant influence on mouthfeel, thanks to their binding to lubricious salivary proteins. Carbonated beverages, such as sparkling wine, beer and fizzy soft drinks, are also characterized by distinctive tastes and a different mouthfeel, in comparison to their non-carbonated, or flat, counterparts. While the taste differences can be partially attributed to the sourness of the dissolved carbonic acid, this does not account for the mouthfeel effects, and here we enter the realm of soft tribology.
A multidisciplinary, multinational team consisting of Sorin-Cristian Vlădescu, Sophie Bozorgi, Songtao Hu, Stefan K. Baier, Connor Myant, Guy Carpenter and Tom Reddyhoff (from Imperial College London, King’s College London Dental Institute, Shanghai Jiao Tong University, Pepsi-Co and the University of Queensland) have recently addressed this problem in a study
2 involving monitoring friction and bubble behavior in model mouth contacts lubricated by carbonated fluids. To imitate the mouth, they used a glass hemisphere rubbing against a silicone-rubber (PDMS) disk, in a pin-on-disk configuration, equipped with a fluorescence-microscopy system for imaging the contact. They added human saliva in some experiments, optionally dyed to enable the adsorbed films to be imaged.
An experiment carried out with carbonated and non-carbonated water in the model mouth showed a striking difference in friction with and without CO
2 addition
(see Figure 1).
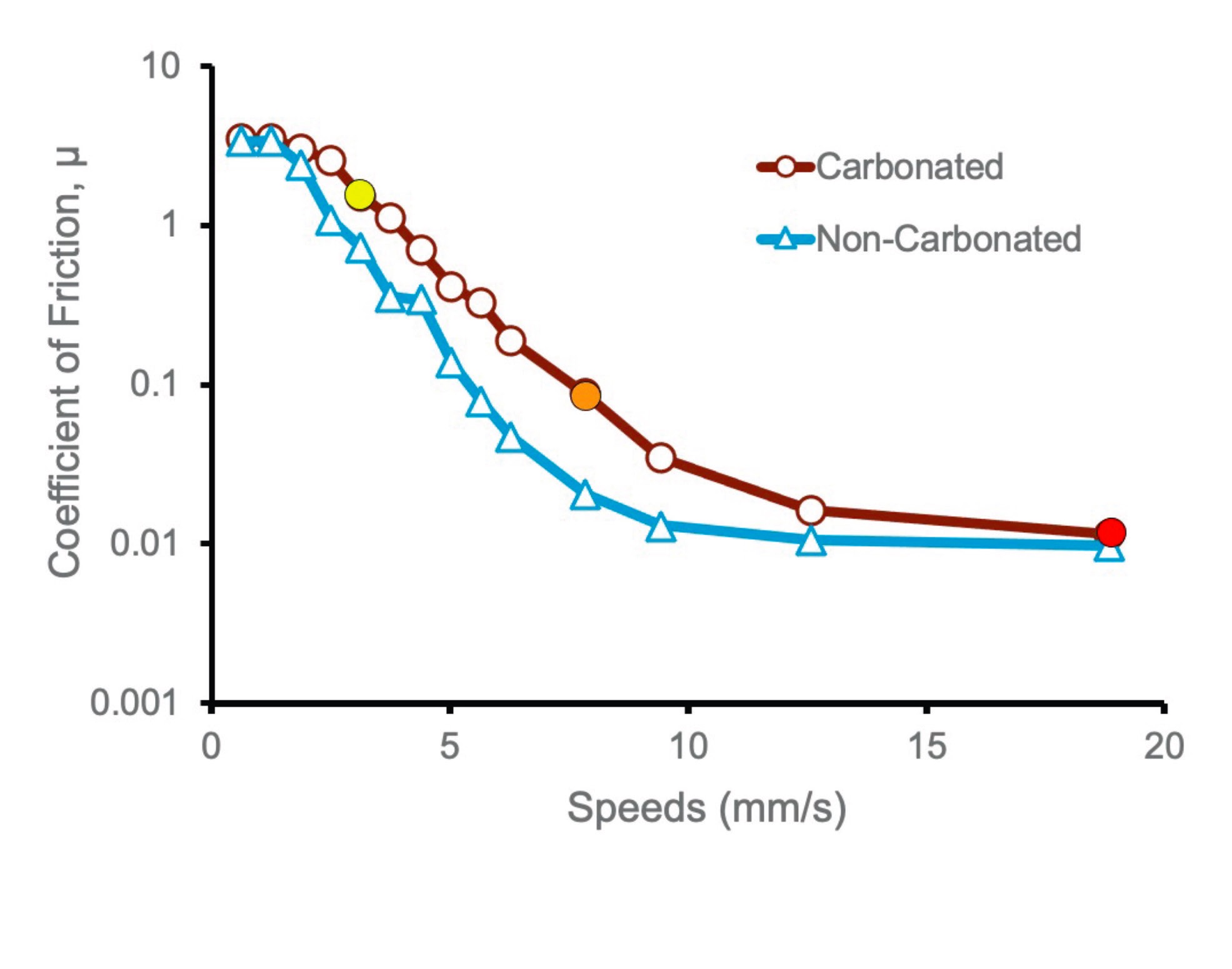
Figure 1. Friction versus speed curve for model-mouth contact (0.2 N load) lubricated with carbonated and non-carbonated deionized water (no saliva present). By kind permission from Ref. 2.
It is notable that the difference occurs only in the mixed-lubrication regime, the curves coinciding in both the boundary regime and at the onset of hydrodynamic lubrication. This implies that the differences caused by carbonation are neither due to adhesion effects (or the boundary-regime values would be different), nor viscosity changes (or the higher-speed values would be different) but are likely the result of differences in water-film thickness, leading to an increase in surface contact.
By adding dye to the water and monitoring the contact with fluorescence microscopy, this hypothesis could be tested
(see Figure 2), and, indeed, it was found that CO<sub>2</sub> bubbles were formed at the contact inlet, starving it of water. Moreover, the increase in friction was proportional to the fraction of the inlet that was deprived of water!
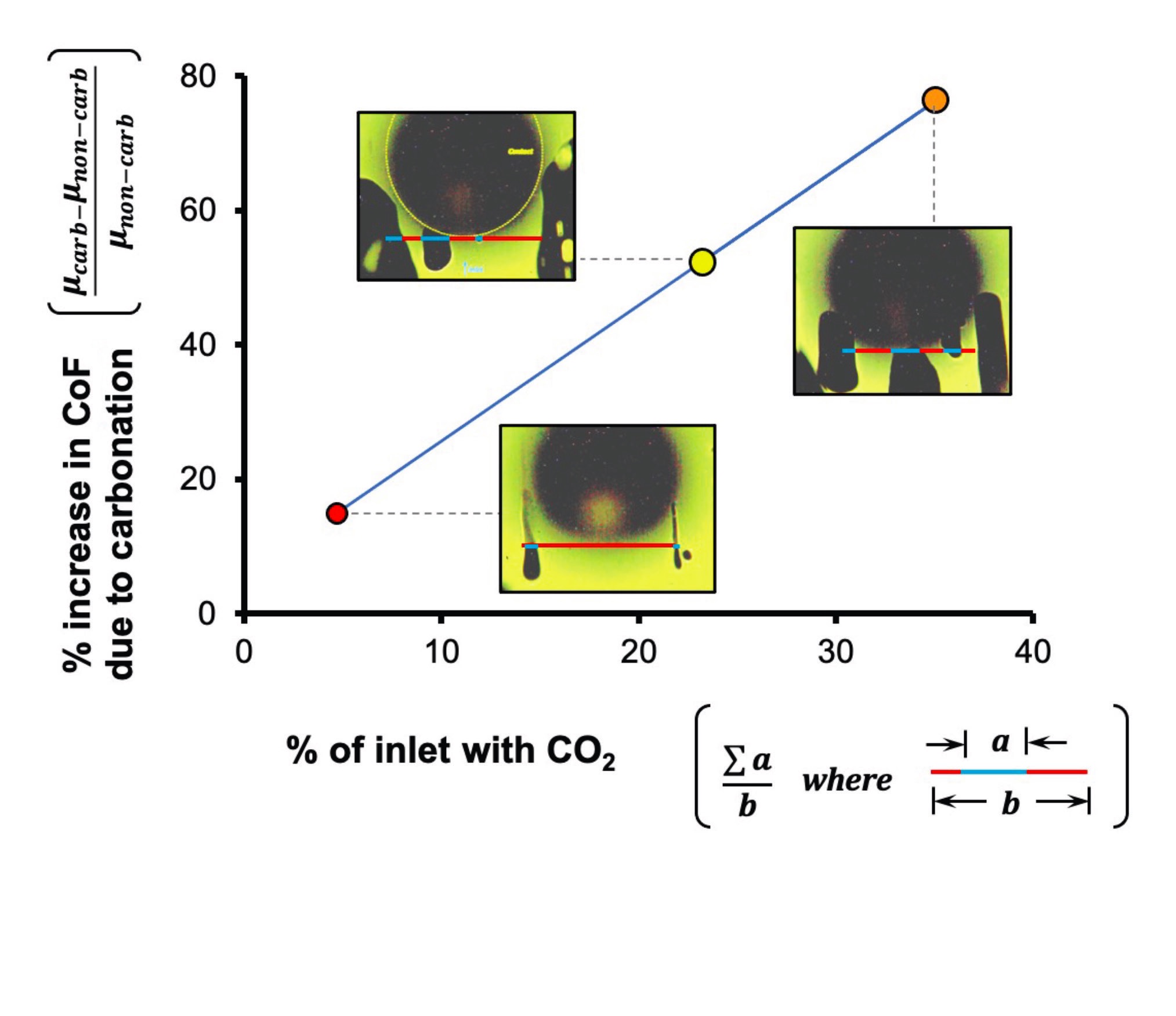 Figure 2. Percent increase in friction (comparing carbonated μcarb, non-carbonated, μnon-carb, friction data from Figure 1) versus percent of inlet that is starved of water (obtained from fluorescence microscopy images). The large, dark circle corresponds to the contact. The other dark shapes are CO2 bubbles. By kind permission from Ref. 2.
Figure 2. Percent increase in friction (comparing carbonated μcarb, non-carbonated, μnon-carb, friction data from Figure 1) versus percent of inlet that is starved of water (obtained from fluorescence microscopy images). The large, dark circle corresponds to the contact. The other dark shapes are CO2 bubbles. By kind permission from Ref. 2.
A further set of tribological tests was run to examine the effect of carbonated water on the adsorbed layer of saliva (the “pellicle”). After running for 10 minutes in pure, fluorescently dyed saliva, carbonated or non-carbonated water was introduced into the contact
(see Figure 3). The fluorescence intensity (corresponding to the amount of adsorbed saliva) and the friction were simultaneously measured. Interestingly, a significantly greater drop in the amount of pellicle occurs upon the addition of carbonated than non-carbonated water. This correlates with a marked rise in friction in the carbonated case.
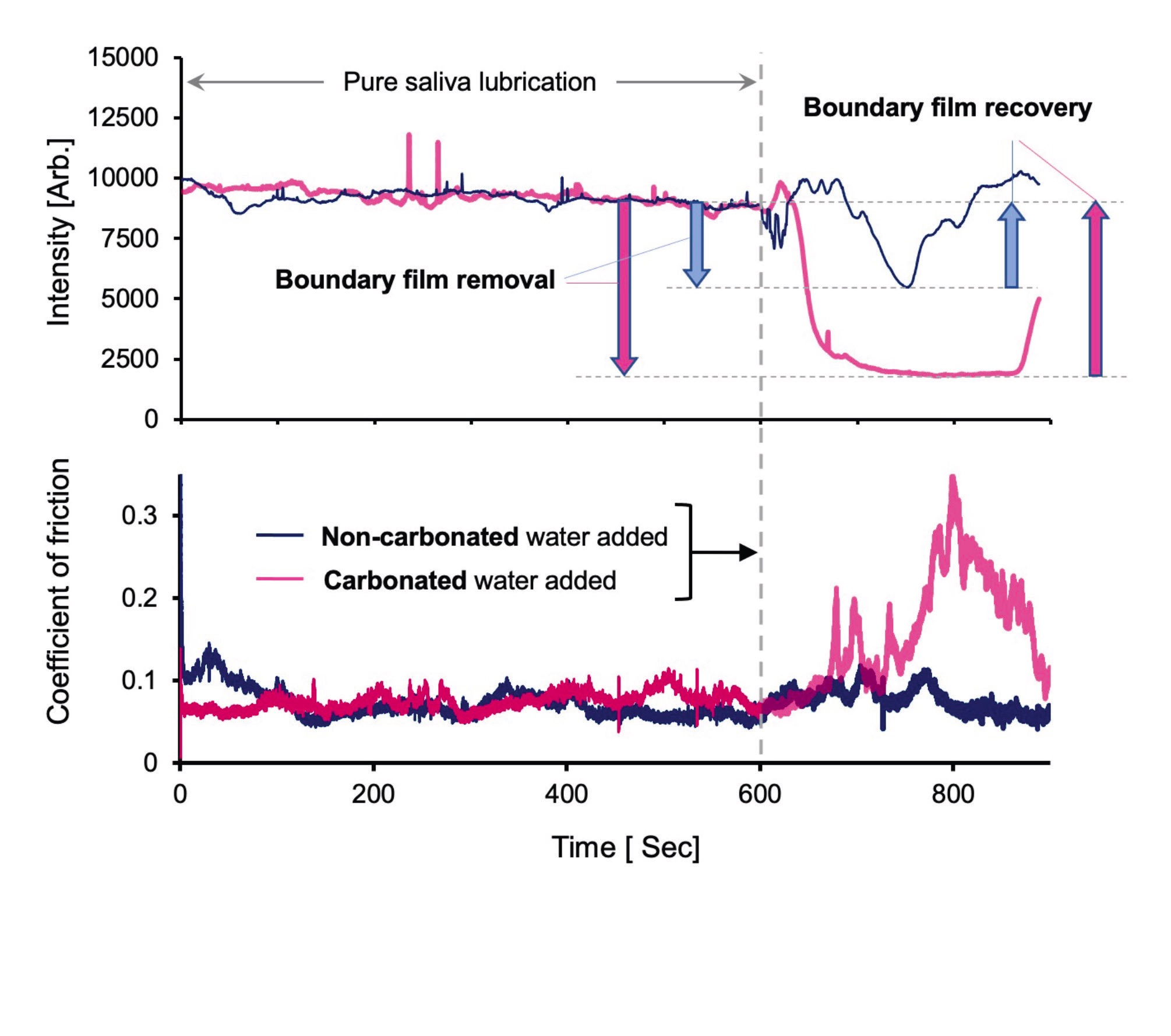 Figure 3. (Top) In-contact fluorescence intensity and (bottom) friction versus time for dyetagged, saliva-lubricated contact in the model mouth, showing effects of addition of carbonated and non-carbonated water. By kind permission from Ref. 2.
Figure 3. (Top) In-contact fluorescence intensity and (bottom) friction versus time for dyetagged, saliva-lubricated contact in the model mouth, showing effects of addition of carbonated and non-carbonated water. By kind permission from Ref. 2.
This removal of the lubricious saliva layer, leading to higher friction in the mouth, is comparable to that associated with the astringency effects of tannins in red wine,
1 where the salivary proteins are being crosslinked by tannin’s polyphenols. In the carbonated-beverage case, it is carbonic acid that removes the saliva layer, either by competing for surface sites or by interacting directly with the salivary proteins.
Thanks to these elegant tribological and imaging experiments, we now know that the mouthfeel of carbonated drinks results from a combination of effects, ranging from the simple blocking of the lubricating water layer by bubbles, to phenomena, which, as with the astringency of red wine, involve more complex interactions with the salivary pellicle.
REFERENCES
1.
Tysoe, W.T. and Spencer, N.D. (2020), “The tribology of red wine,” TLT,
76 (12), pp. 68-70. Available
here.
2.
Vlădescu, S.-C., Bozorgi, S., Hu, S., Baier, S.K., Myant, C., Carpenter, G. and Reddyhoff, T. (2021), “Effects of beverage carbonation on lubrication mechanisms and mouthfeel,”
J Coll Interf Sci, 586, pp. 142-151.
Eddy Tysoe is a distinguished professor of physical chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
Nic Spencer is emeritus professor of surface science and technology at the ETH Zurich, Switzerland, and editor-in-chief of STLE-affiliated Tribology Letters journal. You can reach him at nspencer@ethz.ch.