KEY CONCEPTS
•
A direct method to study the internal structure of a lithium-metal battery was developed that utilizes a femtosecond laser to mill through the battery’s outer metal casing.
•
Evaluation of a lithium-metal battery after 101 cycles under conditions similar to those found in electric vehicles showed that the battery separator was completely shredded, large lithium-metal deposits were present, and the battery had failed.
•
The researchers found no evidence of dendrite formation in the failed battery.
Research is continuing to better understand why lithium-metal batteries fail under high energy density rates after only a few cycles. The motivation for this work is that lithium-metal batteries exhibit 50% higher energy densities than lithium-ion batteries, the current choice for commercial applications such as electric vehicles.
In a previous TLT article,
1 a study was discussed attributing loss of lithium-metal battery performance due to the formation of unreacted lithium metal that is wrapped in the solid electrolyte interphase (SEI) preventing participation in the battery’s charge/discharge cycles. SEI is generated as the battery starts cycling due to the interaction of lithium with the battery’s liquid electrolyte. The salts in the electrolyte appear to isolate the unreacted lithium metal.
Dr. Katharine Harrison, scientist and engineer in Nanoscale Sciences at Sandia National Laboratories in Albuquerque, EditorN.M., says, “The currently accepted failure mechanism for lithium-metal batteries with liquid electrolytes involves the formation of dendrites at the lithium-metal anode that can penetrate the polymer separator between the two electrodes. Eventually, the dendrites reach the cathode causing a short circuit leading to battery failure. But this mechanism is in doubt because the accepted size of dendrites, which is greater than one micron in diameter, cannot possibly move through the nanoscale structure of the polymer separator. The soft metallic nature of lithium metal also makes it difficult to believe that dendrites could pierce the surface of the separator. This puts the current theory in question, particularly since recent cryogenic electron microscopy studies found that dendrites are deflected by the separator.”
In exploring this issue, Harrison and her colleague, Dr. Katherine Jungjohann, scientist and engineer at the Center for Integrated Nanotechnologies at Sandia National Laboratories, decided to image how cell failure occurs in liquid electrolytes with nanoporous separators. But the problem faced by the researchers was that using conventional analytical techniques such as X-ray analysis proved to be ineffective because the battery’s stainless-steel casing decreases imaging resolution.
Jungjohann found an alternative approach that circumvented this problem. She says, “We decided to utilize a femtosecond laser to mill through the battery’s outer metal casing under cryogenic temperature conditions and then follow this up by using scanning electron microscopy (SEM) and X-ray spectroscopy to study the battery’s internal cross section. The low temperature was important to freeze the liquid electrolyte so that it was stabilized for imaging.”
Using this technique, for the first time, the researchers have now been able to study the internal structure of a lithium-metal battery and propose an alternative mechanism to explain why premature failure takes place.
 Lithium-metal batteries exhibit 50% higher energy densities than lithium-ion batteries, the current choice for commercial applications such as electric vehicles.
Lithium-metal batteries exhibit 50% higher energy densities than lithium-ion batteries, the current choice for commercial applications such as electric vehicles.
Tearing of the separator
The researchers prepared half cells of 2032 coin cell batteries that included a 50 micron thick layer of lithium metal on a copper foil counter electrode and a 9 micron copper collector as the working electrode. Placed between the two electrodes was a pair of 25 micron thick separators that contained two polypropylene layers sandwiching an interior polyethylene layer.
The electrolyte used in the coin cell battery was a 2.8 molar solution of lithium bis(fluorosulfonyl) imide in 1,2-dimethoxyethane. Harrison says, “In conducting our experiments, we used an excess of electrolyte in the battery. This is a pretty normal procedure in studying how the battery cycles to ensure full wetting of the separator and because a lot of electrolyte is consumed during cycling. In commercial operation, the electrolyte concentration is kept as low as possible to maintain battery energy density.”
The researchers cycled the battery at a high rate of 1.88 milliamps per square centimeter to simulate conditions that will be found in applications such as electric vehicles. They were able to analyze the inner workings of the battery after each charge/discharge cycle.
After one cycle, the researchers found that SEI formed immediately as the battery started cycling. Included within the SEI was disconnected lithium which, as reported in the previous TLT article,
1 is one reason why lithium-metal batteries fail prematurely.
Harrison says, “As we evaluated the battery cell after 101 cycles, we found that the separator was completely torn by the SEI, and large lithium-metal deposits were present. This leads to a short circuiting of the battery and total failure.”
But the researchers did not notice the presence of any dendrites. Jungjohann says, “We determined that the mechanical properties of the SEI created pathways for the lithium metal to penetrate and tear the plastic separator. It also is possible that hard tips formed in the SEI could pierce the separator.”
Figure 1 shows a scanning electron micrograph of one of the lithium-metal half cells evaluated after a specific experiment. Sections of the battery are divided through color coding into lithium, the separator, SEI/ electrolyte and the copper component of the electrodes. This image clearly shows that the SEI/electrolyte has penetrated the separator.
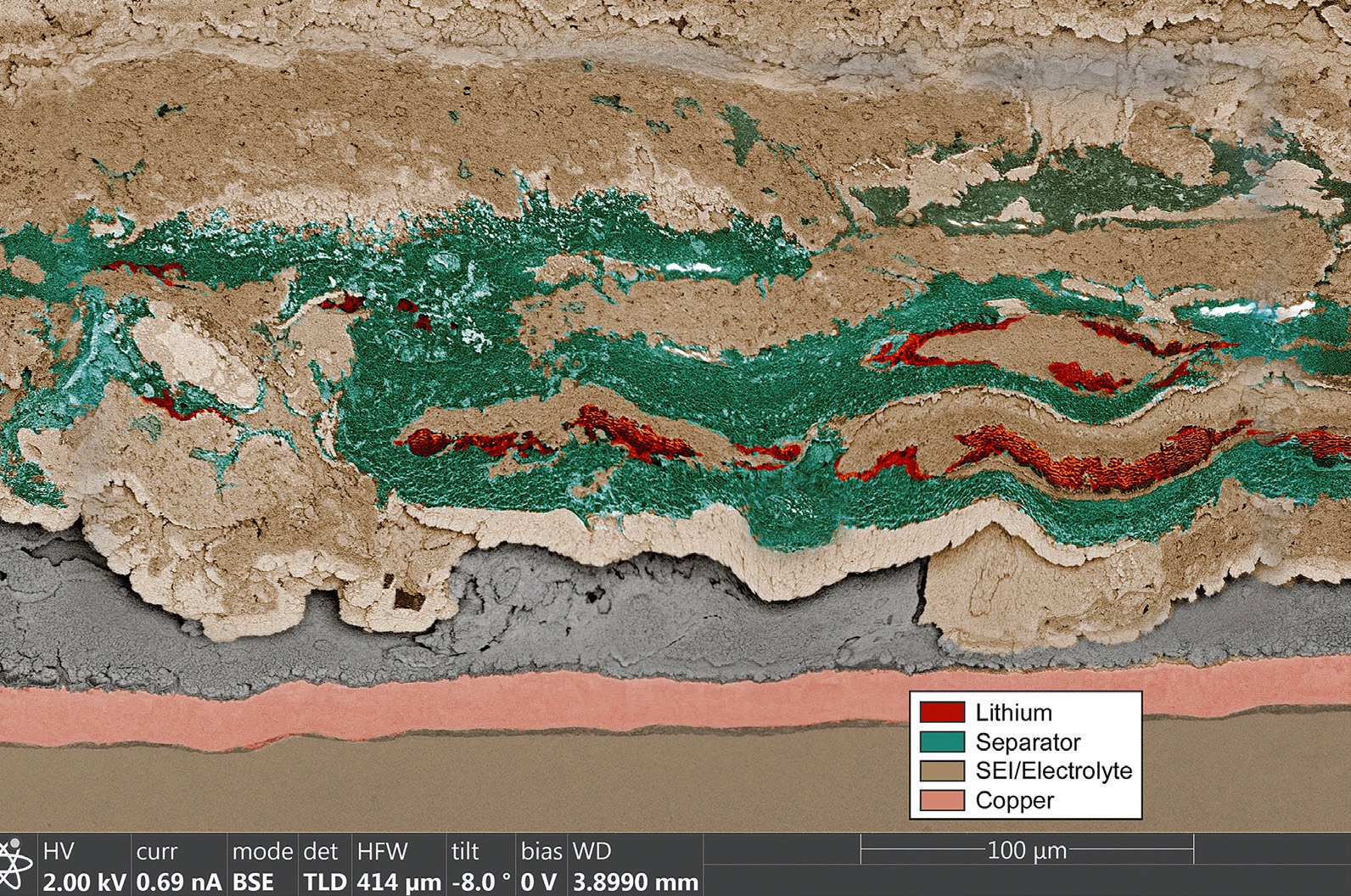 Figure 1. A scanning electron micrograph of a high-rate cycled lithium-metal half cell is shown. The copper present originates from the current collector. Figure courtesy of Sandia National Laboratories.
Figure 1. A scanning electron micrograph of a high-rate cycled lithium-metal half cell is shown. The copper present originates from the current collector. Figure courtesy of Sandia National Laboratories.
With empirical evidence showing this new mechanism for lithium-metal battery failure, the question arises about how to minimize it. Harrison says, “The evidence we have produced suggests that formation of SEI with liquid electrolytes leads to failure, and working with a solid electrolyte is one potential mitigation. Dendrites may form in the solid electrolyte, but at least it does not appear as if the high cycling rate conditions will lead to shredding of the separator, and SEI reactions can be less extensive. Our work also shows that attention must be paid to the composition and structure of the separator when cycling lithium in liquid electrolytes to find an alternative that may not be as vulnerable to tearing under high cycling rate conditions.”
Future work will concern evaluating the inner workings of the battery to better understand the composition of the various species formed during rapid cycling. Harrison says, “We also are evaluating other liquid electrolytes to determine if the same separator tearing phenomenon is observed. External pressure experiments will be conducted to ascertain how changes in pressure will impact the separator. Eventually, we also will evaluate solid electrolytes to determine if they could be a better option.”
Additional information can be found in a recent articles
2 or by contacting Jungjohann at
kljungj@sandia.gov.
REFERENCES
1.
Canter, N. (2019), “Determining how lithium-metal batteries fail,” TLT,
75 (12), pp. 12-13. Available
here.
2.
Jungjohann, K., Gannon, R., Goriparti, S., Randolph, S., Merrill, L., Johnson, D., Zavadil, K., Harris, S. and Harrison, K. (2021), “Cryogenic laser ablation reveals short-circuit mechanism in lithium metal batteries,”
ACS Energy Letters, 6 (6), pp. 2138-2144.