KEY CONCEPTS
•
Petroleum-based lubricants have environmental impacts through their lifecycle.
•
Potential lubricant alternatives include both solid lubricants and environmentally friendly lubricants (EFLs) that offer environmental benefits while still meeting lubricant needs.
•
Improving wear resistance through microstructure design also can help meet engine needs.
Increasing the lifespan of different tribosensitive components while reducing environmental impacts is becoming a more important area of research. A possible approach to solving this issue is the use of solid lubricants to enhance the wear resistance of different components. Another approach is the use of “green or environmental” fluids for enhancing lubrication where removing or decreasing the use of ecotoxic oils for lubrication is the goal. Another answer is by using components that are wear-resistant through enhanced microstructure design.
Solid lubricants
Challenges facing the use of solid lubricants include:
•
Low mechanical strength
•
Limited temperature applicability
•
High application costs.
Andy Nieto, assistant professor at the Naval Postgraduate School in Monterey, Calif., says reducing friction and effectively dissipating frictional heating are key mechanisms in reducing wear. Two-dimensional (2D) nanomaterials are one possible approach for solid lubricants that create protective surface layers as solid-state lubricants.
Nieto provides the example of graphene nanoplatelets (GNPs) as a 2D nanomaterial that offers enhanced wear resistance. Nieto says, “The use of GNPs embedded in ceramic composites has enhanced wear resistance by inducing the formation of a thin, tough and lubricous tribofilm that protects the ceramic from severe wear.”
GNP-reinforced aluminum oxide (Al
2O
3) composites have been formed by spark plasma sintering. Nieto notes, “The highest wear resistance of aluminum oxide is attributed to formation of a continuous, protective and ultrathin graphene tribofilm on the wear surface.” The tribofilm forms “due to the high shear forces induced by countersurface movement and localized heating, which causes GNPs delamination, overlap and welding together.”
1
One area Nieto mentions where GNPs have been used is as reinforcement to alumina ceramics to enhance microscale tribological behavior, which could be beneficial for ceramic-on-ceramic hip transplant applications. The paper notes, “The addition of GNPs lead to improvements in fracture toughness and wear (scratch) resistance of 21% and 39%, respectively.”
2 GNP-induced toughening was considered to be the source of the improved wear resistance in the alumina ceramics. In addition, human osteoblast cells “were observed to survive and proliferate robustly in the GNP-reinforced samples, particularly those with high (10%-15%) GNP content.” In other words, cytotoxicity effects related to the GNPs were not seen.
Nieto says, “2D nanomaterials have great promise as solid-state lubricants due to their excellent mechanical properties, low density, ability to shear along the high surface area basal planes and to generate protective tribofilms under certain tribological loadings.” The most promising materials Nieto notes include “GNPs, boron nitride nanoplatelets (BNNP), tungsten disulfide (WS
2) and the ternary nanolaminated carbides, nitrides (MAX phase) and borides (MAB phases).”
STLE member Stephen Berkebile, research physicist and tribological materials technical area lead at the U.S. Army Combat Capabilities Development Command (DEVCOM) Army Research Laboratory (ARL) in Aberdeen Proving Ground, Md., identifies other potential solid lubricants, beyond graphite or graphene or various ceramics, including:
•
Transition-metal dichalcogenides, molybdenum sulfide (MoS
2) and the previously mentioned tungsten disulfide
•
Hexagonal boron nitride (h-BN)
•
Coinage metals gold, silver and copper
•
Lead
•
Some polymers like polytetrafluoroethylene (PTFE) and fluorinated ethylene propylene (FEP).
STLE member Pradeep Menezes, assistant professor in the mechanical engineering department at the University of Nevada in Reno, Nev., categorizes solid lubricant materials into four categories: organic polymers, lamellar solids, soft materials and oxides and sulfates (
see Table 1).
TABLE 1. SOLID LUBRICANT MATERIALS
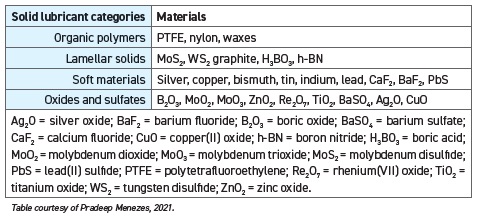
Menezes notes that the organic polymers carry inherent lubricity that can provide lower friction at room temperature; however, these materials suffer from severe wear. Menezes says, “The lamellar solids are the most attractive solid lubricant materials that generate lubricity due to their weak inter-lamella bonds.” The soft material category includes materials with lower shear strength that act as a lubricant at the sliding interface.
The fourth category of metal oxides and sulfates are materials that Menezes notes can “provide lubricity for high-temperature applications,” while combining these oxides “also can yield a complex oxide system with solid lubricating capacity.” Menezes “utilizes the approach of a complex oxide system to generate novel ceramic coatings using supersonic particle deposition” at the Surface Engineering and Tribology Laboratory at the University of Nevada, Reno.
Environmentally friendly lubricants
Nieto notes that GNPs are an example of an environmentally friendly lubricant (EFL) that enhance wear resistance during demanding applications. Nieto says, “GNPs are derived from only carbon, of which there is an abundant supply.” In addition, GNPs also have shown great biocompatibility and no signs of cytotoxicity, making them excellent candidates for green or environmentally friendly materials.
Berkebile notes that while not a fluid, coinage metals (i.e., gold, silver, copper) “are generally environmentally friendly and stable at higher temperatures.” However, he also observes that copper compounds can be toxic.
Berkebile says, “Synthetic base stock fluids (i.e., polyalphaolefin, polyolester) are generally much more environmentally friendly from a toxicity perspective than base stocks distilled from crude oil.” A potential problem is that some additives to these synthetic base stock fluids can increase toxicity. Berkebile also lists zinc dialkyldithiophosphate (ZDDP), which is a highly effective antiwear additive used in engine oils that “is toxic to aquatic wildlife; not biodegradable; can affect catalytic converters; and contains phosphorus, sulfur and zinc, all three of which may form problematic compounds.” To meet EPA requirements for emissions, there has been a push to reduce ZDDP usage.
Menezes notes, “Most lubricants’ lifecycles end in the environment, either by intentional disposal or by unintentional leakage during transportation and usage.” This end result makes minimizing the risks associated with such environmental exposure of lubricants important.
Berkebile provides an example of moving toward solving a toxicity issue and increasing environmental compatibility in tricresyl phosphate (TCP), which is “a highly effective antiwear additive in high-performance gearbox and jet turbine lubricants.” While the ortho isomer of tricresyl has high neurological toxicity, the para and meta isomers have little to no toxicity. When the modern production process is tuned to favor the para and meta isomers, the production of the ortho isomer is reduced; however, more work should be done on this topic.
Berkebile notes that while there has been research into alternatives to TCP, these efforts have concentrated on performance rather than environmental friendliness, as these alternatives also contain phosphate esters (i.e., tributyl phosphate or triphenyl phosphate). A more effective phosphate ester may permit a reduction in phosphate amounts.
 Types of EFL
Types of EFL
EPA considers three factors when defining a lubricant as an environmentally acceptable lubricant (EAL): biodegradability, minimal aquatic toxicity and bioaccumulation.
3 Another term is EFL, which indicates a similar notion and has become an influential marketing term. Menezes says that a lubricant can usually be considered as EFL “if it is partially biodegradable, or if it demonstrates low toxicity, or even if it is partially sourced from biomass or plants.” Menezes lists biolubricant categories as:
1.
Vegetable oils
2.
Synthetic esters
3.
Others.
Synthetic esters can be produced from the esterification of biobased materials. Menezes says a number of synthetic esters are currently available and have been specifically tailored for use in hydraulic oil, stern tube oil, thruster oil, gear lubricant and grease.
Menezes says, “Some petroleum-based oil could be synthesized to incorporate biodegradability and, therefore, be considered as EAL/EFL.” One example of this is polyalkylene glycol, which is produced from the polymerization of ethylene or propylene oxide. Ionic liquid lubricants also have been introduced recently and are partially sourced from biological resources, making them EFL.
Microstructure
Menezes notes, “The design of microstructure with improved wear resistance can be carried out by the incorporation of harder nano- and micron-particles into the matrix.” These harder particles can be oxides and carbides (e.g., aluminum oxide [Al
2O
3], tungsten carbide [WC], silicon carbide [SiC], silicon dioxide [SiO
2], titanium carbide [TiC]).
Menezes noted that the incorporation of fly ash (i.e., a coal combustion product consisting of SiO
2, Al
2O
3 and ferric oxide [Fe
2O
3]) also has been used to improve the wear resistance of metals and alloys. Menezes says, “Other techniques to improve wear resistance are based on surface treatments that enhance the hardness of the surface.” These techniques “alter the surface microstructure and develop refined grains that yield higher surface hardness and wear resistance.” Menezes gives the examples of laser surface processing (LSP), friction stir welding and sandblasting as surface processing techniques that can enhance surface hardness (
see Steel Microstructure).
Steel microstructure
Berkebile provides examples of microstructure that offer enhanced wear resistance in steels:
•
Nanocrystalline microstructure provides enhanced wear resistance through increased hardness.
•
Case-hardening processes in steel like carburizing and hardening can alter near-surface microstructure by introducing residual stresses that aid in both the reduction of wear from high-cycle surface fatigue and the initiation of surface cracks.
•
A quench process can harden steel by creating martensitic microstructure with the resulting retained austenite having a complex relationship to wear performance.
•
Precipitates or segregated inclusions, “such as molybdenum in steel microstructures, can aid in arresting microcracks caused by fatigue; however, impurities in steel can initiate cracks that lead to pitting or other forms of fatigue-based wear.”
Future industry outlook
Nieto notes that while “GNPs are starting to be looked into for technology transfer and transitioned into commercial applications,” a challenge is to develop processing routes that can efficiently incorporate GNPs into bulk form.
Nieto says, “BNNPs have not received as much attention as GNPs; however, the temperature envelope of BNNPs is much greater than GNPs.” For example, BNNPs can be stable in an oxidative environment for temperatures as high as 1,200 C, while GNPs can start to deteriorate in the 600 C to 800 C range.
Berkebile says, “The Military Vehicle Industry Consortium (MVIC) is exploring modernization of fluids for the Department of Defense.” This effort is being directed by the DEVCOM Ground Vehicle Systems Center, a sister organization to ARL under the Army Futures Command.
In order for any lubricant to be considered “green,” Berkebile says the entire lifecycle should be considered, which includes evaluating the amount and toxicity of waste from its production, as well as its ability to be disposed in an environmentally friendly way.
Menezes says, “Global biolubricant market is projected to reach $2.46 billion by 2025, up from $2.20 billion in 2019.” This growth is because many countries are substituting biolubricants for petroleum-based lubricants. Menezes notes that Germany, Italy, the Nordic countries, Benelux (economic union of Belgium, the Netherlands and Luxembourg) and France are actively promoting the use of biobased chemicals. Menezes says, “The increased awareness against global warming has already influenced many consumers to take EFLs, and it is going to increase over the next decades,” leading the industry to potentially experience high growth.
FOR FURTHER READING
Beckman, M. (2019), “Bearing the load,” TLT,
75 (7), pp. 46-51. Available
here.
REFERENCES
1.
Zhang, C., Nieto, A. and Agarwal, A. (2016), “Ultrathin graphene tribofilm formation during wear of Al
2O
3 – graphene composites,”
Nanomaterials and Energy,
5 (1), pp. 1-9, click
here.
2.
Nieto, A., Zhao, J.M., Han, U., Hwang, K. and Schoenung, J.M. (2016), “Microscale tribological behavior and in vitro biocompatibility of graphene platelet reinforced alumina,”
Journal of the mechanical behavior of biomedical materials,
61, pp. 122-134.
3.
EPA (2011),
Environmentally Acceptable Lubricants. Available
here.
Andrea R. Aikin is a freelance science writer and editor based in the Denver area. You can contact her at
pivoaiki@sprynet.com.