KEY CONCEPTS
•
A more sustainable approach has been developed to oxidize methane to methanol at room temperature in an aqueous environment under neutral pH conditions.
•
The researchers determined that a bimetallic catalyst (based on titanium dioxide and copper [III] oxide) was needed to facilitate conversion of methane to methyl but prevent complete oxidation of methyl to carbon dioxide.
•
Titanium dioxide facilitates the conversion of methane to methyl while copper (III) oxide completes the transformation to methanol.
Research is underway to develop more sustainable pathways for the manufacture of materials that can be used as fuels or in the production of synthetic base stocks and additives useful in the formulation of lubricants. One of the key processes under evaluation is the splitting of water into oxygen and hydrogen.
In a previous TLT article,
1 work was published that demonstrated how a key catalyst for the oxygen evolution reaction (OER), which is used in the splitting of water, can be regenerated. The mixed nickel-iron hydroxide catalyst undergoes a phase separation during the reaction reducing its effectiveness. A process known as intermittent electrochemical reduction was discovered that can reverse the phase separation.
A process related to the OER is the manufacture of methanol from methane. This basic hydrocarbon, the main component in natural gas, is typically burned to generate electricity and heat, which leads approximately to the generation of 1 gigaton of carbon dioxide emissions.
Methane also can be oxidized to methanol through a thermocatalytic conversion process. Meenesh Singh, assistant professor of chemical engineering at the University of Illinois at Chicago (UIC) in Chicago, Ill., says, “Thermocatalytic conversion is a high-energy intensive reaction in which natural gas is converted to synthesis gas and then to methanol. Temperatures for the reaction range from 200 C-300 C, and high pressures also are required.”
A more sustainable approach to manufacturing methanol is desirable. Singh believes a logical strategy is to conduct the reaction in an aqueous environment under ambient conditions. He says, “The challenge for converting methane to any other compound is its high stability. The carbon-hydrogen bond in methane exhibits an energy of 439 kilojoules per mole. Energy needs to be introduced to force methane to react, but instead of heat, we propose to apply an electric current in the form of an energy potential. The electricity being supplied is through renewable sources.”
This approach was tried unsuccessfully in the 1980s and also can lead to the conversion of methane to carbon dioxide, which is not desirable. Aditya Prajapati, lead author and a graduate student at UIC, says, “The key to developing a process is to activate the carbon-hydrogen bonds in methane through the presence of transition metal oxides, which provide the source of oxygen. Care must be taken to ensure that the ultimate product is not oxygen because the OER, while necessary, also is a competing process.”
A new approach has now been taken to identify a transition metal oxide catalyst that can successfully convert methane to methanol at room temperature in an aqueous environment.
Bimetallic catalyst
Prajapati initially evaluated the performance of 12 transition metal oxides at neutral and alkaline pH values using a rotating-disk electrode cell. The catalysts were prepared by oxidizing their respective transition metals through the use of linear sweep voltammetry, which placed an oxide layer on each substrate.
Singh says, “We avoided acidic pH because most metal catalysts are not stable under those conditions. Neutral pH is preferred over basic pH because the high concentration of hydroxyl ions, in the latter case, can cause the oxidation reaction to produce oxygen and not methanol. The presence of oxygen interferes with the key catalytic site, which is present on top of the metal oxide catalyst.”
Under neutral pH conditions, four transition metal oxides (based on iridium, titanium, lead and platinum) display activity in methane oxidation. Singh says, “In using reactant-impulse chronoamperometry, we determined the binding energy of methane to the four metal oxide catalysts. As part of this phase of the study, we found that the binding energy is higher and the electrostatic potential for the four-metal oxide catalyst lower than the inactive catalysts. This lower electrostatic potential facilitates binding of methane followed by its activation for complete oxidation to carbon dioxide.”
The challenge for the researchers was to isolate methanol and stop the process short of producing carbon dioxide. Singh says, “Complicating this issue is that the methane oxidation reaction interacts with an intermediate step in the OER. This means that the OER is an essential aspect of oxidizing methane.”
Open-circuit measurement identified the presence of methanol in titanium dioxide catalyzed reactions under higher 35potential. Singh says, “The titanium dioxide systematically converted methane to lower fragments of CH
x (x = 1,2,3) with the primary species being *CH
3. A second catalyst is needed to prevent titanium dioxide from over oxidizing methanol to carbon dioxide.”
The researchers turned to copper (III) oxide because this transition metal oxide facilitates methanol formation but not complete oxidation to carbon dioxide. A bimetallic catalyst (based on titanium dioxide and copper [III] oxide) was prepared by electrodepositing 10% copper by weight on titanium metal followed by oxidation. In this configuration, titanium metal oxide oxidizes methane to methyl, which is then converted to methanol by copper (III) oxide (
see Figure 3).
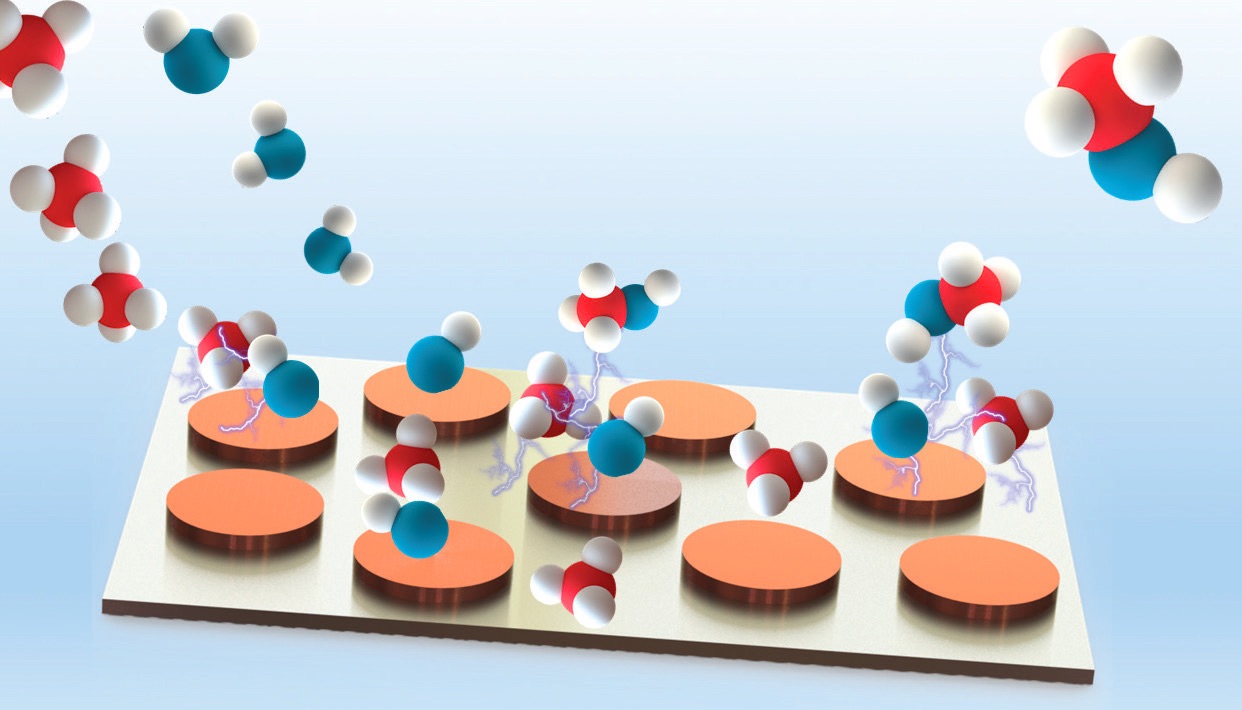 Figure 3. This schematic shows the conversion of methane to methanol under mild aqueous conditions using a bimetallic catalyst. Carbon atoms are shown in orange, hydrogen atoms are in white, and oxygen atoms are in blue. Figure courtesy of University of Illinois at Chicago.
Figure 3. This schematic shows the conversion of methane to methanol under mild aqueous conditions using a bimetallic catalyst. Carbon atoms are shown in orange, hydrogen atoms are in white, and oxygen atoms are in blue. Figure courtesy of University of Illinois at Chicago.
Singh says, “Future work will involve adjusting the ratio of titanium to copper to optimize the formation of methanol. We also will be increasing the amount of the planar catalyst in an attempt to increase the yield of and conversion to methanol.”
Additional information on this work can be found in a recent article
2 or by contacting Singh at
mrsingh@uic.edu.
REFERENCES
1.
Canter, N. (2021), “Regeneration of water splitting catalyst,” TLT,
77 (1), pp. 16-17.
2.
Prajapati, A., Collins, B., Goodpaster, J. and Singh, M. (2021), “Fundamental insight into electrochemical oxidation of methane towards methanol on transition metal oxides,”
Proceedings of the National Academy of Sciences, 118 (8), e2023233118.