KEY CONCEPTS
•
Borophene displays superior strength, flexibility and conductivity compared to graphene but is not stable under ambient conditions.
•
Hydrogenation of borophene produced borophane, which is more stable as demonstrated by being two orders of magnitude less susceptible to oxidation.
•
Thermal annealing of borophane produces borophene, which suggests that this additive may perform in a similar manner to a classic extreme-pressure lubricant additive.
Identification of new materials suitable as lubricants is an ongoing process. There continues to be a need because applications are becoming more challenging. Lubricants are required to provide long-term performance in machinery that is operating under extreme temperature and pressure conditions.
For these types of applications, solid lubricants are desirable. Graphite, which is composed of layers or planes of carbon atoms, is a well-known solid lubricant. In a previous TLT article,
1 a two-dimensional single layer of carbon atoms known as graphene was isolated and found to be the strongest material ever examined at that time. Graphene has been used as an additive to reduce friction and wear in lubricants.
A second class of solid lubricants are based on boron. Examples include boron nitride and boric acid. A previous TLT article
2 described the synthesis of the ionic compound, boron boride, that is organized into two nanoclusters in a single crystalline phase. This material is very similar in features to diamond.
Boron atoms also can organize into atomically thin layers in a similar manner to graphene. Mark Hersam, Walter P. Murphy professor of materials science and engineering at Northwestern University in Evanston, Ill., says, “Borophene is a term defining a family of two-dimensional boron polymorphs. Due to the electron deficiency of boron, borophene cannot adopt the same hexagonal atomic lattice of graphene. Instead, boron forms a triangular lattice with a periodic array of vacancies that appear as hollow hexagons.”
As a true metal, borophene differs in properties with graphene. Hersam says, “Borophene exhibits superior strength, flexibility and conductivity compared to graphene, which suggests that this material has the potential to revolutionize applications such as batteries and photovoltaics. Theoretically, borophene also has the potential to function as a high temperature superconductor. The reason is the transition temperature for superconductivity has an inverse relationship with mass. Since boron is one of the lightest elements, it is anticipated to have unusually high temperature superconductivity. A compound known as magnesium diboride also exhibits superconductivity, where the boron structure is similar to borophene.”
The problem with borophene is the material’s instability under ambient conditions. Hersam says, “Unfortunately, borophene rapidly oxidizes in the presence of air and can only be used in an ultrahigh vacuum chamber, where water and oxygen are excluded.”
This strategy is not practical, and efforts to use chemical passivation have only been met with limited success. A new approach has now been found to convert borophene into a derivative that is stable under ambient conditions.
Hydrogenation
Hersam and his colleagues synthesized a more stable derivative known as borophane by hydrogenation of borophene. He says, “We decided to try hydrogenation based on previous work with silicon that reduced its chemical reactivity following hydrogen passivation. In a similar manner, we found that borophane was less susceptible to oxidation by two orders of magnitude compared to borophene.” The researchers found that borophane did not oxidize after exposure to ambient conditions for one week through the use of X-ray photoelectron spectroscopy.
Borophene was initially produced on clean silver substrates under ultrahigh vacuum conditions through elemental boron evaporation. Atomic hydrogen converted borophene to borophane at room temperature. A hot tungsten filament maintained at 1,600 C was used to produce atomic hydrogen from molecular hydrogen.
At least eight distinct borophane polymorphs were found in the hydrogenated material. There are subtle differences among these structures, but all are stable at ambient temperature. The polymorph present in the highest concentration was designated as v
1/6-30º borophane. Hersam says, “The ѵ
1/6 term indicates the number of boron atom vacancies. For this polymorph, one-sixth of boron atoms are missing from the triangular lattice. The 30º represents the angle of the boron lattice compared to the underlying silver substrate.”
Using a combination of spectroscopy and modeling, the researchers determined that v
1/6-30º borophane contains a combination of two-center-two-electron boron-hydrogen bonds and three-center-two-electron boron-hydrogen-boron bonds. The latter are known as “banana” bonds due to their shape.
The modeling analysis uses density functional theory to help determine the arrangement of the boron and hydrogen atoms. Hersam says, “While microscopy provided us with an indication of the structure of this borophane polymorph, the images left some ambiguity. Modeling led us to conclude that borophane has the structure shown in Figure 1, where boron atoms are shown in teal, and hydrogen atoms are in red.”
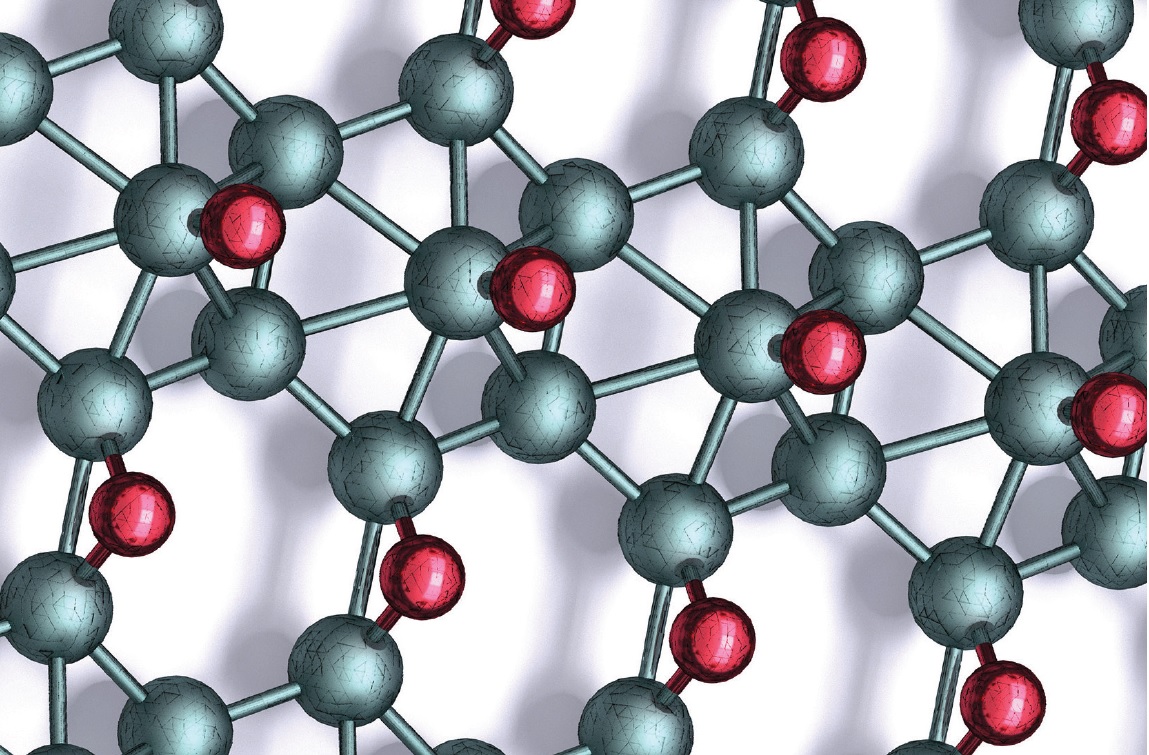
Figure 1. A schematic based on modeling analysis shows the structure of borophane, which is an ambient temperature stable derivative of borophene that has potential for use as a lubricant additive and in batteries. The boron atoms are in teal, and the hydrogen atoms are in red. Figure courtesy of Northwestern University.
The researchers are still exploring the detailed mechanisms for why hydrogenation significantly improves the stability of this two-dimensional layer of boron atoms. Hersam says, “One strategy for reducing the reactivity of boron is to chemically tie up its bonds as we have done through adding hydrogen.”
One other intriguing property about borophane is that it will revert to borophene upon thermal annealing at 300 C. Hersam says, “The boron-hydrogen bonds are not thermally stable at elevated temperatures.”
This result suggests that borophane may perform in a similar manner to classic extreme-pressure lubricant additives such as sulfurized compounds that activate at high temperature. Further work may determine whether borophane is a potential new solid lubricant.
Hersam says, “Knowing that derivatization of borophene is needed to improve stability, we will next be exploring other chemical modification schemes for borophene in an effort to achieve even better performance than borophane.”
Additional information on this research can be found in a recent article
3 or by contacting Hersam at
m-hersam@northwestern.edu.
REFERENCES
1.
Canter, N. (2009), “Graphene: The strongest material ever examined,” TLT,
65 (2), pp. 28-29.
2.
Canter, N. (2009), “Boron-based ionic compound,” TLT,
65 (5), pp. 14-15.
3.
Li, Q., Kolluru, V., Rahn, M., Schwenker, E., Li, S., Hennig, R., Darancet, P., Chan, M. and Hersam, M. (2021), “Synthesis of borophane polymorphs through hydrogenation of borophene,”
Science, 371 (6534), pp. 1143-1148.