KEY CONCEPTS
•
A high viscosity index is desirable for a lubricant in many applications, so many suppliers have attempted to “monetize” VI.
•
As the average VI increases between API Groups I, II and III, the concept “higher numbered base oil groups are better” is prevalent.
•
The roots of base oils grouping began in a purely technical cross-industry exercise.
Viscosity index (VI) is described by Wikipedia as “an arbitrary, unit-less measure of a fluid’s change in viscosity relative to temperature change.”
1 However, arbitrary and unit-less does not equate to a lack of value. As a technical description of the viscosity-temperature relationship of a lubricating fluid, it has great technical value.
A high VI fluid can have a lower viscosity at low temperatures or a higher viscosity at high temperatures or both, relative to an isoviscous fluid at an intermediate temperature. This can bring multiple benefits. Lower viscosity at low temperatures allows flow, so cold-start for vehicles or operation of industrial equipment in cold climates is facilitated by a flowing lubricant, rather than metal-on-metal contact causing damage. A higher viscosity at high temperatures results in a thicker oil film, maintaining the hydrodynamic regime to higher temperatures or lower sliding speeds and protecting parts by, again, preventing contact.
But there are other benefits, both real and implicit, which are strongly linked to the chemistry of the fluids. When considering only hydrocarbons (base oils and PAOs), the molecules in higher VI fluids tend to be more linear, and the size distribution is more uniform. These molecular properties can be used to demonstrate or imply benefits in lubricants.
Higher VI base stocks also can reduce the amount of viscosity modifier needed in multigrade oils, which can result in lower material costs and shorter (and therefore cheaper) blending times. Thus, a higher VI can be “monetized” through more sales, a higher selling price or both. For end-users of the finished oils, less viscosity modifier can equate to cleaner equipment, as some viscosity modifiers can contribute to the formation of sludge, lacquer or varnish as they degrade in service. Therefore, higher VI can be sold as a means toward a “cleaner” formulation.
Many of these benefits have their foundations in the differences between the chemistry of the American Petroleum Institute (API) base oils groups, so examining why these marketing claims are so prevalent begins with the definition of the API base oils groups.
Base oils interchange
Until the early 1990s, there were only two types of base oil—paraffinic and naphthenic. But this was unsatisfactory for all stakeholders. Philip Reeve, director of UK-based ADLU Consultancy, was with Exxon Chemical (Paramins) at the time. “The expectations of additive suppliers were that the classification of base oils would help reduce the amount of engine testing required to qualify lubricants using similar base oil types,” he says. This expectation also was true for many of the oil companies that produced their own formulations for mainstream lubricants and sourced a variety of base oils. The automotive OEMs wanted to ensure that there would be no lubricant-related warranty claims with associated reputational damage.
So, the aim was to establish a set of base oils groups that allowed a defined mix of additives to be blended into a variety of different base oils and produce a lubricant that met a set of performance criteria. Thus, testing costs would be lowered, but industrywide data would support that the mix of additives in base oil A would pass all the necessary rig and engine tests (and therefore not damage vehicles) because that mix of additives had passed all required tests in base oil B.
The bodies concerned were SAE International, the American Automotive Manufacturers Association (later to become ILSAC when Japanese OEMs joined), API and the Chemical Manufacturer’s Association (now the American Chemistry Council, ACC). In 1990 they produced the Engine Oil Licensing and Certification System (EOLCS), otherwise known as API 1509.
2
Industry consultant Steve Swedberg, who at the time chaired SAE Technical Committee 1 – Engine Oils, explains, “After the general introduction of hydroprocessed oils, it became apparent that there were significant differences [in performance in automotive crankcase applications] between solvent-treated and hydrogen-processed paraffinic base oils. As a part of the EOLCS development, a task group was formed to try to address the problem. The first iteration of the base oil groups [in 1992] was Group I – solvent extracted oils, Group II – hydrogen processed oil and Group III – everything else.”
The original proposed definitions of base oils groups dealt only with the chemistry of the fluids. The proposed Group I had less than 90% saturates and/or more than 0.03% sulfur, while the proposed Group II had 90% or more saturates and 0.03% or less sulfur. All other base stocks, including synthetics, not included in these groups were placed in Group III. According to industry consultant Amy Claxton (now of IHS Markit), presenting at the ICIS World Base Oils and Lubricants Conference in London in February 2020, the refiners disliked the idea of defining products by the process.
Returning to 1992-1993, Swedberg recalls that a parallel discussion began in SAE Technical Committee 1 about how to classify synthetics. He says, “Shell and BP presented data showing that severely hydrogen-processed oils had similar engine test properties to PAOs. The debate was about whether or not a severely refined petroleum product is essentially the same as a synthesized product. BP won the argument on the basis that they could either refine or take the wax formed and react it to make the same product.” The VI of these base oils was >120, so significantly different from the norm for commercially produced hydroprocessed oils in North America, which was in the range of 95-105.
It should be noted that “synthetic,” when used for crankcase lubricants in many countries, is now predominantly a marketing term rather than a technical one.
Before the debate concluded on whether severely hydroprocessed oils could be called synthetic, the base stocks task force decided that base oil groups would be defined by their properties. At this point, viscosity index entered the properties table, which is unchanged in 2020 (
see Table 1).
Table 1. API base oils groups
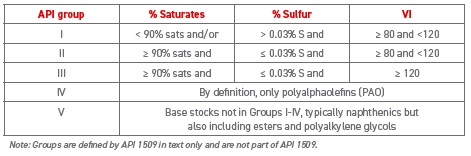
The table allowed the germination of the idea that “higher base oil group number is better,” which is broadly, but not universally, true. The definition of “better” depends strongly on the application. Jeff Biamonte, global product development manager for ExxonMobil Basestocks and Specialties, says, “It’s important to look at what viscosity the equipment design requires and what the operating temperatures are for that equipment. Equipment design dictates the necessary lubricant viscosity range that will provide enough lubricant film thickness to reduce friction and wear. If equipment is run in a controlled narrow operating temperature range, an oil with a higher VI might not provide any additional value over a lower VI oil (
see It’s Not All VI).”
It’s not all VI
Tim Smith of Lubrizol reported at the 2019 STLE Annual Meeting in Nashville, Tenn., that energy losses in earthmoving equipment could not be explained by the relative viscosity indices of the fluids being studied.
A Smith and colleagues found that flow in the pipes was affected by the viscosity modifier (VM) used and that this was not related to the VI of the fluid. A polymethacrylate VM of a type commonly used in energy efficient hydraulic fluids, and which gave a higher VI fluid, produced more turbulent flow than a fluid based on a novel hydrocarbon VM. The latter fluid had already been shown to be significantly more energy efficient in field trials and rig tests, despite the lower VI.
A. Moon, M. (October 2019), “Efficiency flows from polymer VM,” Lubes’n’Greases, pp. 34-38.
Other factors might influence the choice of lubricant. For example, marine cylinder lubricants, which lubricate the engines of most large ocean-going tankers and container ships, were almost exclusively based on the superior solvency of API Group I base oils well into the 2010s. These oils are usually SAE 50 grade, so the kinematic viscosity at 100 C has to be be- tween 16.3 and 21.9 mm
2/s, which is too high for API Group II or Group III base fluids alone (
see The Evolution of +).
The evolution of +
Lubricants refining is a fast-evolving business. In 1992-1993, when the API base oils groups were created, API Group III had no members that were available commercially. Shell and BP were either consuming their very high viscosity index oils internally or selling into captive markets. The value of VI to these companies was that they could blend 5W-xx motor oils without using expensive PAO. Several Japanese automotive OEMs were specifying and purchasing 5W-xx fluids for factory-fill oils.
When marketing to formulators of automotive lubricants, the differences between API Groups II, III and IV are often reduced to the relationship between the low temperature properties and volatility of fluids that are isoviscous at 100 C. API Group IV base oils (PAOs) have the lowest volatility and the best low-temperature properties, whether measured by pour point or some low-temperature flow test. Group II base oils usually have the highest volatility, as they have more small molecules and the worst low-temperature properties, as they also have more molecules with high mass. API Group III base oils sit in the middle and could demand a premium price relative to API Group II while closing the pricing gap on PAO.
While Group II producers could simply have increased the degree of hydrofinishing to produce a higher VI base stock, they were faced with loss of yield. Therefore, it took around a decade of catalyst and process optimization before Group II manufacturers had the capability to consistently produce base oils at the top end of the VI range in sufficiently high yields that made their production cost effective.
It also was important to differentiate their products from other API Group II base oils in the minds of existing and potential customers, so by the end of the 20th Century, the unofficial Group II+ designation arrived in the market.
B Most marketers utilizing this term would use it for a fluid with a VI >110, but some understand the term to mean VI ≥115.
The value of these fluids to some of the larger customers was that they could “trim” formulations at the blending plant to meet the exacting demands of major OEMs for factory fill oils without using additional viscosity modifier or more expensive PAO, if a batch of base oil was delivered still in specification, but with a low VI. Additional polymer can lead to engine deposits, so raising the VI by use of Group II+ fluids can be pitched as a cleanliness improvement.
Leaping forward another decade, improvements have been made in API Group III base oils manufacture, such that it is possible to blend 0W-xx formulations, previously the province of PAO. Norman Sheppard of Bahrain Petroleum Co. (BAPCO) says, “Continuous technological advances in the catalysts we use for hydroprocessing and ISO dewaxing technologies are critical to matching our base oil product quality to sophisticated market needs. Group III base oils such as ours are now capable of blending the increasingly demanded lower-viscosity automotive lubricants, without need of correction fluids such as costly PAO. This has opened up new cost-effective possibilities for lube blenders and OEMs.”
Some suppliers label their products Group III+. Again, this is an unofficial designation; it is based on VI, often quoted as >130, but there is not universal agreement on the criterion.
B. Petro-Canada HDEO, Henderson, H., Mack, P., Steckle, W. and Swinney, B. (1998), “Higher quality base oils for tomorrow’s engine oil performance categories,” SAE Technical Paper 982582. Available at https://doi.org/10.4271/982582.
Property or process?
Despite the focus on properties in the API classification, a common perception grew that:
“Group I is solvent-extracted, Group II is hydroprocessed/hydrotreated, and Group III is (hydrotreated and) hydroisomerized.”
Whether based on process or property, the creation of these base oil groups meant that the additives suppliers and oil companies were able to demonstrate acceptable performance in a wider variety of base oils utilizing fewer engine tests and, therefore, reducing cost. (At that time, many oil companies blended crankcase formulations from individual components, rather than additives packages, so were as active as the additives suppliers in running standard engine tests.)
In parallel, Swedberg explains that the CMA “was able to develop a detailed additive development process that complements the EOLCS. It included test registration, minor modifications to additive formulations and other heretofore undocumented procedures employed by the additive industry.” Reeve confirms that the new protocols for formulation development brought a greater discipline to the process and created a more level playing field for participants.
Higher is better
It wasn’t just in passenger car motor oils where the advantages of formulations based on API Group II base oils were being deployed. Dr. Peter Smith, who developed industrial lubricants for both Castrol (now BP) and Shell, takes up the story. He says, “Early in the century, industrial turbine lubricants for gas, steam or combined cycle power stations began to reach the market based on API Group II or III base oils. Of course, the API designation had less direct relevance in industrial applications, as approvals were often made by OEMs on a case-by-case basis, often with limited or no base oil read-across being allowed, but it was a useful shorthand.”
These turbine lubricants had much longer lifetimes than their predecessors, when treated with the correct additives. So, the base oils were often promoted as being more thermally, oxidatively or hydrolytically stable than the less paraffinic (API Group I) base oils that had preceded them.
The sales pitch was simple and went something like:
“The hydrocarbons in Group I base oils contain ring structures and significant branching; these contribute to both low viscosity index and poor oxidative stability.” While this is true regarding hydrocarbons only, it ignored that some of the sulfur- and nitrogen-containing components of Group I base oils are natural antioxidants, and that some base oils manufacturers could retain those “good” species during Group I manufacture, while removing those species (also sulfur- and nitrogen-containing) that were pro-oxidation. It also is true that the average Group II base oils on the market at that time were more oxidatively stable than the average Group I in most parts of the world. As a result, the sales pitch became undisputed “fact,” despite the correlation not being 100%.
In some cases, the correlation between structure and performance was demonstrated. Chevron, for example, charted viscosity index and oxidative stability in the late 1990s for Group II and Group III base oils (
see Figure 1). The structure/activity relationship here was much more valid, as all fluids contained less impurities than Group I base oils. However, even at this juncture, it can be seen that the 120 VI fluid sits well below the curve.
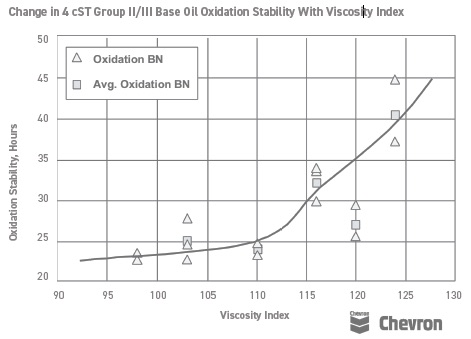 Figure 1. Chevron charted viscosity index and oxidative stability in the late 1990s for Group II and Group III base oils. Figure courtesy of Chevron.
Figure 1. Chevron charted viscosity index and oxidative stability in the late 1990s for Group II and Group III base oils. Figure courtesy of Chevron.
As a result, the link between viscosity index and oxidation stability was established in customer minds, and as the VI increased in sequence from API Group I through to API Group IV, the market was prepared to believe that oxidation stability always followed VI.
There also were secondary effects in turbine oils that were a function of VI, as Smith explains. He says, “The lubricants in a gas turbine can operate at temperatures approaching or exceeding 80-90 C in critical parts of the lubrication system such as the bearings, but the ISO grade is based on the kinematic viscosity at 40 C. The higher VI of Group II and III-type base oils made it possible to show end-users that an ISO 32 grade could have comparable or higher viscosity at 90 C than the ISO 46 fluid they were using based on more conventional (API Group I) base oils.”
“Where gas turbines are being used for peak energy generation with many cold starts, the lower viscosity at lower temperatures was very attractive to customers, as less of the calorific value of the combusted gas was likely to be wasted to overcome losses due to the fluid (
see Figure 2).”
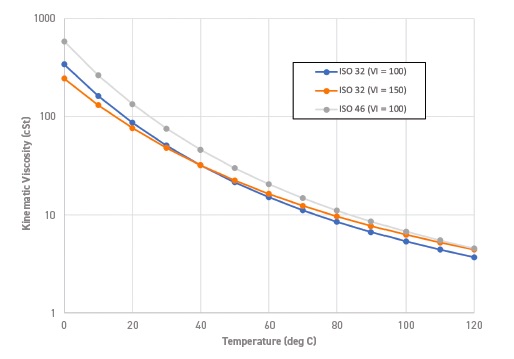 Figure 2. Kinematic viscosity versus temperature. Figure courtesy of professor Ian Taylor.
VI: Panacea?
Figure 2. Kinematic viscosity versus temperature. Figure courtesy of professor Ian Taylor.
VI: Panacea?
Some people in the lubricants business who sell high VI fluids (base oils to lubricants blenders or lubricants marketers to end-users) would have their customers believe that VI is the means by which all fluids should be measured, as it is a proxy for so much else. While this can be true, Biamonte speaks for all the experts who contributed to this article: “There is a constant desire to define what is ‘good’ and ‘bad’—but really the answer is always ‘it depends.’ Too often, single properties are used to judge performance of a base stock—this is not a recommended approach. When selecting a base stock for lubricant design, the focus needs to be on the application, what the operating conditions are and the benefits that the finished lubricant will provide to the customer.”
REFERENCES
1.
Click
here.
2.
Click
here.
STLE member Trevor Gauntlett is a freelance writer and consultant on lubricants based in Wirral, UK. You can contact him at trevor@gauntlettconsulting.co.uk.