KEY CONCEPTS
•
Researchers examined the local structure of the oxygen evolution reaction catalyst strontium iridate to better understand how its structure changes during the process.
•
The stoichiometry of the catalyst changed during the reaction and, even though strontium atoms left the surface during the reaction, sufficient strontium remains to ensure that the catalyst remained active.
•
A structural conversion of the catalyst occurred during the reaction as the shape of the strontium iridate transformed eventually into an amorphous octahedron.
The movement to a hydrogen economy where this element can be used as a power source is ongoing. Fuel cells development is linked directly to how effectively hydrogen can be produced through splitting of water.
The key step in this process is known as the oxygen evolution reaction (OER) where an oxygen anion is oxidized to a neutral oxygen atom. Zhenxing Feng, assistant professor of chemical engineering at Oregon State University in Corvallis, Ore., says, “The challenge in efficiently catalyzing OER is the need to transfer four electrons. This is a much higher barrier to conquer, which requires more energy than the hydrogen evolution reaction. The latter involves the transfer of two electrons.”
In a previous TLT article,
1 researchers developed an approach for regenerating a mixed nickel-iron hydroxide OER catalyst. This catalyst underwent a phase segregation, which involves the formation of a secondary catalyst phase that is partitioned from the primary phase. Application of a low reductive voltage improves the environment around the catalyst’s surface through an intermittent electrochemical reduction reversing the phase segregation.
Currently used OER catalysts such as iridium and ruthenium oxides have demonstrated potential. Feng says, “This catalyst type is based on precious metals but does not exhibit good cost performance. One other concern from past studies was that these oxide catalysts undergo a structural transformation during the OER.”
In most reactions, the catalyst might change structurally during the process due to an interaction with a reactant substrate. But the catalyst will revert back to its original state once the reaction is completed and will be ready to repeat its role in the reaction. Feng says, “Work done with oxide catalysts has shown they will change structure during the OER. The catalyst loses surface area, which leads to deactivation and eventually decomposition. This makes it very challenging to find a suitable OER catalyst.”
The new catalyst used in Feng’s work is strontium iridate, a special type of oxide called perovskite oxide. “This catalyst exhibits 1,000 times better activity in the OER reaction than iridium oxide,” Feng says. “Unfortunately, in a manner similar to other oxide catalysts, strontium iridate also becomes more disordered through an amorphization process in the OER, but interestingly, its activity gets better and then stabilizes in acidic electrolyte condition.”
To better understand the mechanism, Feng and his colleagues decided to examine the OER process using strontium iridate. The researchers studied how the structure of strontium iridate changed during the OER using several analytical techniques.
Amorphous layer
The researchers used surface X-ray scattering and spectroscopy techniques to study changes in the structure, oxidation state and composition of a strontium iridate film with a thickness of 16 nanometers. The OER reaction was initiated under acidic conditions in a 0.1 molar solution of perchloric acid.
Feng says, “After only 15 minutes, we observed the formation of an amorphous layer with a thickness of 2.3 nanometers and the reduction of the crystalline layer by approximately the same amount. The net result was that the overall film thickness of strontium iridate did not change, but there was a significant change in the catalyst’s structure.”
Further testing for up to four hours showed that catalyst performance improved due to an increase in OER current even though the amorphous layer stopped growing. Feng says, “We found that the strontium iridate catalyst did not lose activity but became even more effective.”
The researchers used X-ray absorption spectroscopy and X-ray photoelectron spectroscopy to study how the composition of the amorphous catalyst over the four-hour OER reaction changed. Feng says, “By using this approach, we were able to analyze the local structure on the atomic scale even though long-range analysis was not possible due to the amorphous nature of the catalyst surface.”
This analysis indicated that oxygen atoms from the original strontium iridate crystal lattice were lost along with the loss of strontium atoms and a change in the oxidation state of iridium atoms (from +3 to +4) near the surface of the catalyst. Feng says, “The net effect is a change in the stoichiometry of the catalyst, but, most importantly, sufficient strontium remained in the amorphous and crystalline layers to ensure that the catalyst was not converted to iridium oxide. The change in the iridium oxidation state was done to maintain charge neutrality by compensating for the loss of strontium atoms. At the same time, the oxygen vacancy sites in the lattice are filled, restoring coordination between oxygen and iridium atoms in the lattice.”
The movement of ions into and out of the amorphous structure led to a conversion of the crystalline strontium iridate structure into an amorphous square-planar configuration to compensate for the loss of lattice oxygen. Restoration of lattice oxygen and loss of strontium atoms produced a second transformation into an amorphous octahedral structure that becomes increasingly disordered as the OER reaction proceeds.
This mechanism for how the amorphous layer changes in structure is shown in Figure 3.
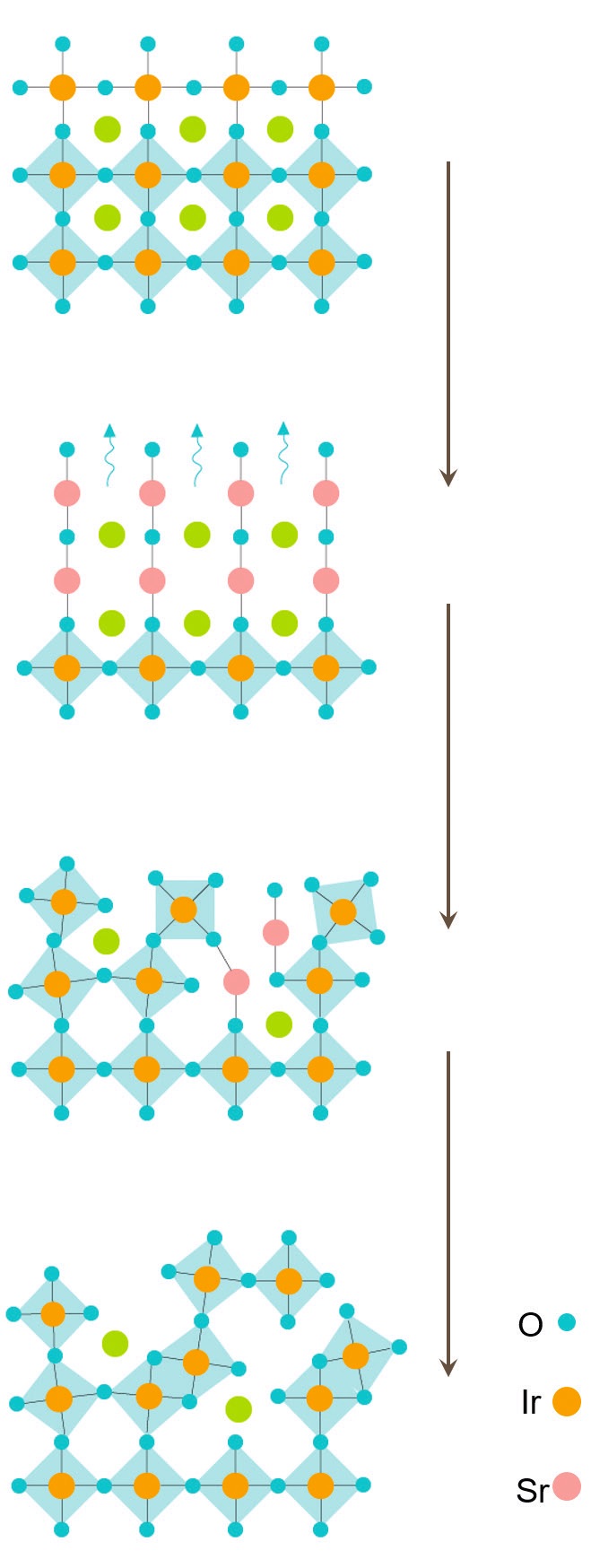 Figure 3. The sequential structural change of the strontium iridate catalyst during OER is shown, starting with the original crystalline octahedral structure on the top and progressing down to the amorphous octahedron on the bottom. Green atoms represent oxygen, orange atoms represent iridium, and pink atoms represent strontium. Figure courtesy of Oregon State University.
Figure 3. The sequential structural change of the strontium iridate catalyst during OER is shown, starting with the original crystalline octahedral structure on the top and progressing down to the amorphous octahedron on the bottom. Green atoms represent oxygen, orange atoms represent iridium, and pink atoms represent strontium. Figure courtesy of Oregon State University.
The work conducted to better understand the strong performance of strontium iridate as an OER catalyst will lead to the development of additional candidates. Feng says, “We will be using the findings of this study to design catalysts that have the stoichiometry of the catalyst once the amorphous layer was generated. Hopefully, this will lead to more active catalysts. Research also will be conducted to identify and evaluate catalysts based on cheaper raw materials (such as iron) than iridium.”
Additional information on this research can be found in a recent article
2 or by contacting Feng at
zhenxing.feng@oregonstate.edu.
REFERENCES
1.
Canter, N. (2021), “Regeneration of water splitting catalyst,” TLT,
77 (1), pp. 16-17.
2.
Wan, G., Freeland, J., Kloppenburg, J., Petretto, G., Nelson, J., Kuo, D., Sun, C., Wen, J., Diulus, T., Herman, G., Dong, Y., Kou, R., Sun, J., Chen, S., Shen, K., Schlom D., Rignanese, G., Hautier, G., Fong, D., Feng, Z., Zhou, H. and Suntivich, J. (2021), “Amorphization mechanism of SrIrO3 electrocatalyst: How oxygen redox initiates ionic diffusion and structural reorganization,”
Science Advances, 7 (2), :eabc7323.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.