KEY CONCEPTS
•
Carbon spheres are spherically shaped structures composed entirely of carbon that have shown promise for capturing carbon dioxide.
•
Pyrolysis of pyromellitic acid at high temperatures under an argon atmosphere produces high porosity carbon spheres with an average diameter of 200 nanometers.
•
Initial testing showed that the carbon spheres selectively adsorbed carbon dioxide and are particularly effective at low pressures.
A key driver moving the development of electric vehicles, wind power and solar energy is the desire to reduce emissions and improve sustainability. This trend is anticipated not only to improve the environment but also the health of individuals.
In a previous TLT article,
1 a new chemistry climate model determined the benefits of using electric vehicles in the U.S. on emissions and the impact on health. Researchers conducting this study found that adoption of 25% electric vehicles in the U.S. light-duty passenger car fleet will lead to a savings of $17 billion in damages attributed to air pollution, health and climate changes annually. This figure rises to $70 billion if the market share of electric vehicles increases to 75%.
Carbon capture technology is increasingly of interest as a strategy to reduce the level of carbon dioxide in the atmosphere. One technology that is under development for use to capture carbon dioxide is carbon spheres.
Dr. Saeid Khodabakhshi, COFUND Fellow of the Energy Safety Research Institute at Swansea University in Swansea, UK, says, “Carbon spheres are spherically shaped structures composed entirely of carbon. The smallest types have a diameter of 1 nanometer and are either amorphous or crystalline in structure. The smallest type of carbon sphere is fullerene, which contains 60 carbon atoms oriented in a sp2 bonding fashion.”
Carbon spheres have been considered to be a substrate that can capture carbon dioxide for some time. Khodabakhshi says, “Many approaches have been developed for the synthesis of carbon spheres, but they are mainly expensive and impractical. Carbon black is a potential source because it contains crystalline graphite plane but has limitations due to inferior gas porosity and the need to be manufactured through the use of combustion.”
Another popular approach for capturing carbon dioxide is the use of aqueous amine solutions. Khodabakhshi says, “While this method is low cost, it is still expensive and leads to the use of corrosive materials. Besides amines, sodium and potassium hydroxide can be used and these alkaline materials need to be neutralized with mineral acids, leading to extra processing steps adding cost.”
The ideal carbon sphere to be used for carbon dioxide capture must not only be small in diameter (around 200 nanometers is desirable) but in pore size to exhibit good selectivity in capturing carbon dioxide in compositions such as the flue gas byproduct of combustion, according to Khodabakhshi.
A new approach for synthesizing carbon spheres that are effective in capturing carbon dioxide and is sustainable in nature is needed. Such an approach has now been developed.
Chemical vapor deposition
The researchers used a method known as chemical vapor deposition (CVD) to produce carbon spheres that exhibit excellent carbon dioxide capture properties. Khodabakhshi says, “Traditional techniques for using CVD to manufacture carbon spheres involve the use of toxic and/or flammable raw materials. Our approach relied on the use of one of the least expensive benzene carboxylic acid derivatives.”
The researchers pyrolyzed terephthalic acid, trimesic acid and pyromellitic acid at temperatures between 600-800 C under an argon atmosphere. Porous carbon spheres were only produced with pyromellitic acid.
A one-step process was used to produce carbon spheres. Khodabakhshi says, “We isolated deposits specifically from the pyrolysis of pyromellitic acid that were determined by scanning transmission electron microscope to be carbon spheres with an average diameter of 200 nanometers (
see Figure 2). The optimum reaction conditions were to heat pyromellitic acid to a temperature of 800 C.”
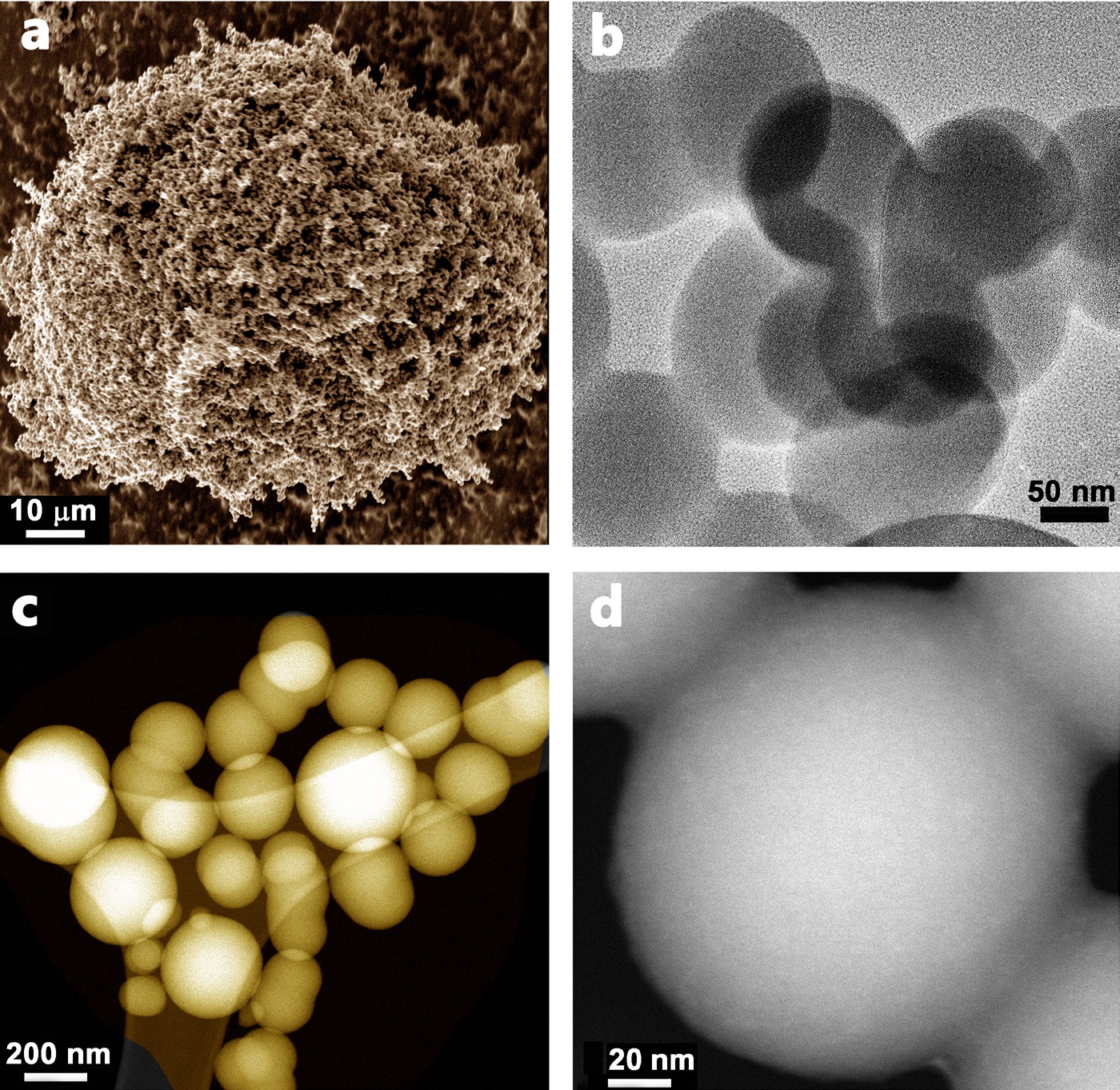 Figure 2. Porous carbon spheres are shown that exhibit the ability to capture carbon dioxide and are derived from a low-cost, readily available feedstock that can be prepared in an environmentally friendly manner. Figure courtesy of Swansea University.
Figure 2. Porous carbon spheres are shown that exhibit the ability to capture carbon dioxide and are derived from a low-cost, readily available feedstock that can be prepared in an environmentally friendly manner. Figure courtesy of Swansea University.
Khodabakhshi estimates that the carbon spheres isolated represent a distribution of carbon atoms.
Benzene carboxylic acid derivatives were used because they are sources of oxygen. As the temperature is increased, the release of carbon dioxide through a decarboxylation process acted as an activator for pore formation in carbon spheres, according to Khodabakhshi. This method eliminates the need for alkali metals, which are currently used for developing porosity in activated carbon production. One indication for why pyromellitic acid was the proper starting material is that the researchers detected the intermediate, pyromellitic dianhydride, is formed during the pyrolysis.
Khodabakhshi says, “We determined pyromellitic anhydride was present from characterization of the pyrolysis reaction mixture. This intermediate was isolated and subjected to the identical reaction conditions to produce carbon spheres with identical physical properties.”
Another advantage of this process is that a silica-based template or unrecyclable metal catalysts are typically needed during the CVD and hydrothermal process or as a substrate for the carbon spheres. Khodabakhshi says, “We found that no template is required because the carbon spheres form through self-assembly.”
Carbon dioxide adsorption testing demonstrated the strong performance of the carbon spheres, particularly at low pressures due to the presence of ultramicropores. This enables the microspheres to selectively adsorb carbon dioxide, which is particularly useful in removing this material from a mixture such as flue gas.
The hydrophobicity of the carbon spheres was evaluated through contact angle measurements and found to be superhydrophobic. Khodabakhshi says, “This result is very important because it indicates that water should not interfere with the capture of carbon dioxide. The carbon spheres prepared from pyromellitic acid should be very effective in adsorbing carbon dioxide in a high humidity environment.”
An important characteristic for using this approach to manufacture carbon spheres is sustainability. Pyromellitic acid is low-cost, readily available feedstock that can be prepared in an environmentally friendly manner.
The researchers are in the process of scaling up the process to make commercial quantities of the carbon spheres. Additional information can be found in a recent article
2 or by contacting Khodabakhshi at
saeid.khodabakhshi@swansea.ac.uk or his co-author, Dr. Enrico Andreoli, at
e.anderoli@swansea.ac.uk.
REFERENCES
1.
Canter, N. (2020), “How will electric vehicle use affect air quality and the health of individuals?,” TLT,
76 (12), pp. 18-19.
2.
Khodabakhshi, S., Kiani, S., Niu, Y., White, A., Suwaileh, W., Palmer, R., Barron, A. and Andreoli, E. (2021), “Facile and environmentally friendly synthesis of ultramicroporous carbon spheres: A significant improvement in CVD method,”
Carbon, 171, pp. 426-436.