KEY CONCEPTS
•
NMR and scanning electron microscopy were conducted to determine how alkali metal additives (such as potassium) improved the performance of lithium-metal batteries.
•
NMR analysis indicated that introduction of potassium ions produced less SEI and a lower concentration of byproducts that can hinder lithium-metal battery performance.
•
SEI formation appears to be reduced because potassium ions minimize decomposition of electrolytes at the lithium-metal anode.
Lithium-metal batteries are under evaluation as a potential energy source for use in applications such as electric vehicles. In contrast to lithium-ion batteries, a metal anode is utilized to take advantage of lithium’s high energy density and low weight.
But safety limitations have been found in working with lithium-metal batteries that can be traced to the formation of dendrites growing from the lithium anode as the battery cycles. These branched structures, composed of unreacted lithium, reduce the performance of the battery and can eventually produce a short circuit if they reach the cathode. A battery short circuit can potentially lead to a fire.
In a previous TLT article,
1 researchers conducted a study to determine how lithium-metal batteries lose performance. The cause was found to probably be the formation of unreacted lithium metal. The reason for the presence of unreacted lithium metal can be traced to the presence of a solid electrolyte interphase (SEI) that forms as the battery starts to operate. The researchers found that the unreacted lithium metal was wrapped in the SEI in the presence of lithium carbonate and lithium oxide electrolyte salts, leaving it unable to participate in the battery cycling.
Lauren Marbella, assistant professor of chemical engineering at Columbia University’s School of Engineering and Applied Science in New York, N.Y., says, “Stabilizing lithium-metal batteries by not growing dendrites is a top priority to improve the performance of applications such as electric vehicles, where range anxiety is making this type of automobile unattractive to consumers.”
From Marbella’s perspective, changing the chemical makeup of the SEI or altering the thickness of the SEI are the best approaches for achieving this goal. She says, “The SEI forms on the electrode surface and interacts with both the lithium-metal anode and the electrolyte. It contains breakdown products due to the high reactivity of lithium metal.”
Work has been done in an attempt to reduce or stabilize the SEI through changing the anion of the lithium salt in the electrolyte, increasing the concentration of the electrolyte and using sacrificial organic electrolyte additives. Some benefits were found, but none of these approaches dealt with the cation.
Addition of other alkali metal additives, such as those based on potassium, cesium and rubidium to the liquid electrolyte, led to improved lithium-metal battery performance. Hypotheses were made that these alkali metal additives thinned the SEI layer, produced electrostatic shielding and generated different chemical species in the SEI.
To determine the effect of alkali metal additives in the electrolyte, a detailed analysis of the interphase between the SEI and the lithium electrode has to be made. Such an analysis has now been conducted.
NMR
Marbella and her colleagues used nuclear magnetic resonance (NMR) to gain insight into how ions based on the alkali metal, potassium, are able to facilitate ion flow at the lithium anode, electrolyte interface. She says, “We decided to work with potassium because this alkali metal is the only commercially viable option. Sodium does not work, and cesium and rubidium are too expensive.”
The researchers conducted their study by adding potassium hexafluorophosphate at various concentrations to a 1 molar solution of lithium hexafluorophosphate in an electrolyte composed of a 1:1 ratio of ethylene carbonate and dimethyl carbonate. This electrolyte composition is widely used in lithium-metal batteries.
Before doing the NMR analysis, the researchers used scanning electron microscopy to examine the microstructure at the lithium anode/electrolyte interface. The left image (red background) in Figure 2 shows high-surface area lithium microstructures after plating on the anode. Once potassium hexafluorophosphate was added, more smooth, rounded lithium microstructures with lower surface areas were formed as shown in the right image (purple background) in Figure 2. This indicated that the potassium salt was influencing the microstructure.
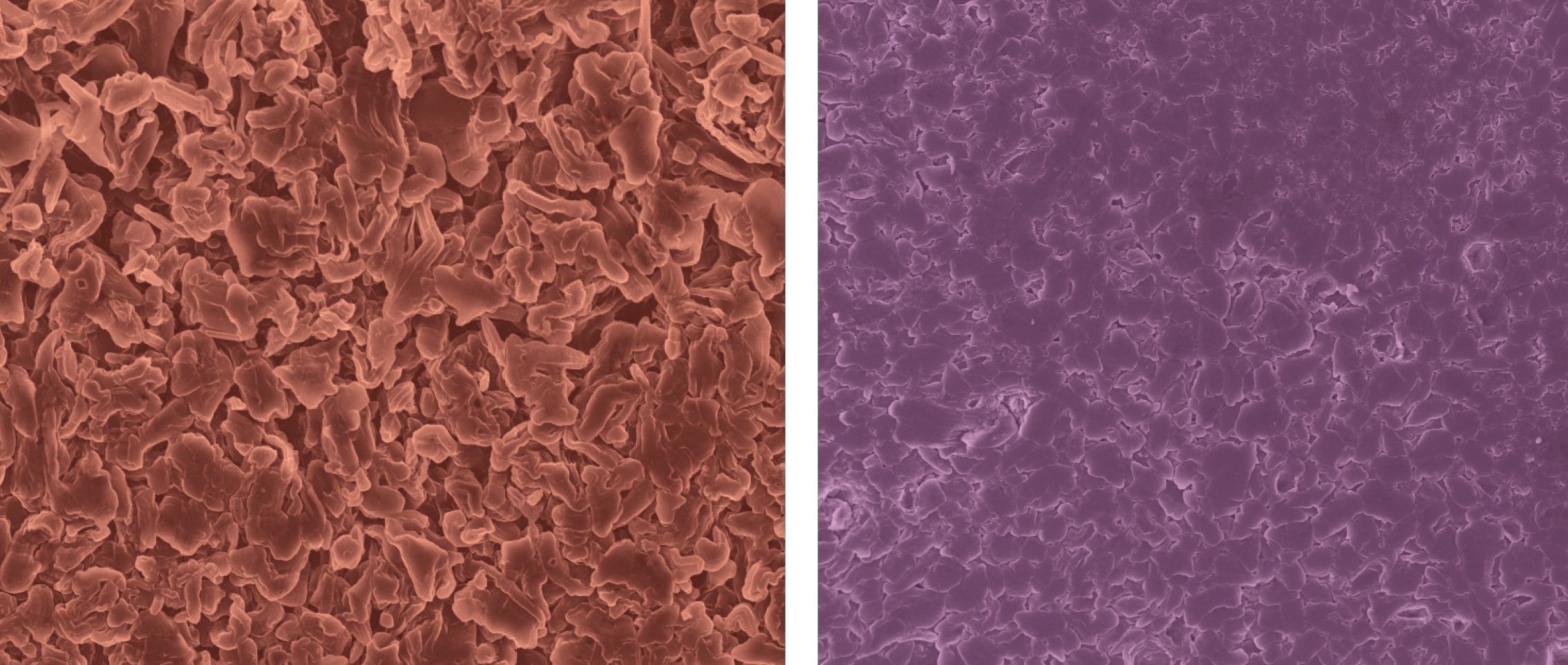 Figure 2. Addition of an alkali metal additive to the electrolyte converts high-surface area lithium microstructures (left image with the red background) to more smooth, rounded lithium microstructures with lower surface areas (right image with the purple background). Figure courtesy of Columbia University.
Figure 2. Addition of an alkali metal additive to the electrolyte converts high-surface area lithium microstructures (left image with the red background) to more smooth, rounded lithium microstructures with lower surface areas (right image with the purple background). Figure courtesy of Columbia University.
Marbella says, “We used solution and solid-state NMR to characterize the composition of the electrolyte solution and the components in the SEI. The latter was very challenging to do as we had to use a magic angle spinning technique at a frequency over 10 kilohertz to obtain high-resolution NMR spectra. Achieving this was difficult because lithium is metallic and can generate eddy currents that will stop the spinning. Potassium bromide was added to dilute the sample and minimize any issues with spinning.”
The two NMR techniques showed that introduction of potassium ions leads directly to the formation of less SEI and lower concentrations of byproducts such as lithium carbonate and soluble organic components such as polyethylene oxide, which have been shown to reduce lithium-metal battery performance.
The researchers turned to density functional theory to better understand why potassium ions reduce electrolyte decomposition. Potassium ions reduce the formation of SEI by minimizing the decomposition of ethylene carbonate and dimethyl carbonate at the lithium-metal anode. As a result, a thinner SEI is present that facilitates lithium ion flow to the metal anode.
Future work will involve determining how potassium ions can interact with electrolytes modified with species, such as fluorinated ethylene carbonate that are under evaluation to boost performance. Marbella says, “We believe that potassium ions provide the opportunity to tune the electrolyte composition to maximize battery performance.”
Additional information can be found in a recent article
2 or by contacting Marbella at
lem2221@columbia.edu.
REFERENCES
1.
Canter, N. (2019), “Determining how lithium-metal batteries fail,” TLT,
75 (12), pp. 12-13.
2.
May, R., Zhang, Y., Denny, S., Viswanathan, V. and Marbella, L. (2020), “Leveraging cation identity to engineer solid electrolyte interphases for rechargeable lithium metal anodes,”
Cell Reports Physical Science, DOI: https://doi.org/10.1016/j.xcrp.2020.100239.