TLT: You both have quite disparate backgrounds and are continents apart, yet have ended up collaborating long distance on FTIR AN lubricant analysis.
van de Voort: Yes, I am a food chemist by training, having specialized in developing quantitative FTIR methods for the analysis of edible oils, which, through an association with Thermal-Lube Inc., evolved into applying those analytical concepts to lubricant condition monitoring (CM), specifically high-speed, high-throughput AN and BN analyses.
Viset: I have spent most of my professional career in the lubricant sector, was aware of Fred’s work and felt that it could have application beyond turnkey systems used by commercial CM laboratories. I contacted him and asked him to look into a simpler means of facilitating manual FTIR AN analysis, but generically, adaptable to basically any FTIR spectrometer.
TLT: You specify quantitative AN FTIR analysis. How does this differ from conventional FTIR CM?
van de Voort: With conventional CM, the analytical protocol defined by ASTM E2412, FTIR spectra are measured on neat oil samples. These are commonly considered to be fingerprints of an oil, which have bands associated with moisture, nitration, oxidation, additives, soot, glycol and the like. With quantitative stoichiometric FTIR, we added a spectrally active basic reagent to the oil and measure proportionate loss of the reagent due to the acid-base reaction (
see Figure 1).
 Figure 1. Continuous oil analysis and treatment (COAT) system suited for high-throughput stoichiometric AN and BN analysis as well as conventional FTIR lubricant CM. Figure courtesy of Thermal-Lube, Montreal, Canada.
Viset:
Figure 1. Continuous oil analysis and treatment (COAT) system suited for high-throughput stoichiometric AN and BN analysis as well as conventional FTIR lubricant CM. Figure courtesy of Thermal-Lube, Montreal, Canada.
Viset: The conventional FTIR measurements carried out by used oil labs are done directly on neat oils and look for spectral variations indicative of changes in the functional groups of constituents making up the oil, its additives and contaminants. However, there are all sorts of interferences and interactions that make interpretation a problem. In quantitative reagent FTIR, you add a reagent to the oil, and then you measure the loss of the reagent by measuring the spectrum before and after the reaction, the spectral change being proportional to the acidity. It’s quite different from conventional FTIR CM, even though you use the same instrument. You are measuring peak heights in a spectrum rather than the volume of a titrant, but otherwise it is much like the conventional ASTM potentiometric titrations, but the stoichiometry is simple, and you don’t need to rely on chemometrics or any fancy statistics to obtain a result.
TLT: What convinced you to work on the AN project together?
van de Voort: Michael is an insistent and persuasive guy, but what triggered my interest was his bringing to my attention SpectraGryph, a generic spectroscopy software package developed in Germany by Dr. Friedrich Menges. Designed to work with the spectral data from almost any FTIR spectrometer, it uses elegant and very simple prepackaged macros to extract, manipulate and process spectral data, its output being a simple .csv or comma separated file. This standard file format is readily accessed by macro-enabled Excel to further process the results to calibrate or predict AN and, ultimately, pass the results onto an LIMS.
Viset: Fred did not take that much convincing; I think he relishes in the intellectual challenge, and he had access to the fancy software to work things out. I just might have found a piece of a puzzle, SpectraGryph, which turned out to be a game changer.
TLT: Why a stoichiometric FTIR AN method? Are there no other FTIR methods that can deliver similar data?
Viset: Well, not really, not in a low-volume sample field situation. In the established industry FTIR methods, e.g., ASTM E2412, using lab IR and FTIRs, these analyses are mostly performed on the neat oils. That makes it simple, but the value of the result also is limited. Some device-specific methods are enhanced with spectral libraries and background correction, correlations and chemometrics approximating or estimating AN values but far from providing accurate and determinative data equaling titrimetric methods. One requires a chemical reaction specific to acidity if you want to get close to equaling the conventional ASTM D664 titrimetric method.
van de Voort: Yes. The fact that Michael was experienced, technically capable and willing to do the experimental work was a clincher—he had access to an Agilent 5500 FTIR equipped with a unique accessory, the TumblIR®, which I could see intuitively would be a godsend in terms of sample handling (
see Figure 2).
 Figure 2. Applying a reacted oil sample onto the TumblIR® ZnSe accessory for analysis is simple, convenient and fast; just wipe clean between samples.
Figure 2. Applying a reacted oil sample onto the TumblIR® ZnSe accessory for analysis is simple, convenient and fast; just wipe clean between samples.
TLT: What is so unique about sample handling?
van de Voort: Well, it is an open architecture IR accessory that allows one to simply put a drop of sample on the ZnSe plate, rotate the accessory head so that it contacts the liquid applied, the IR beam then passing through without an air gap interrupting the beam. This means that CO
2 and moisture vapor interferences are nullified, and the amount of sample that needs to be prepared is minimal, in the order of ~1.5 ml. The TumblIR® accessory is not a necessity, and a conventional IR transmission cell loaded by aspiration also will do but requires somewhat more sample to be prepared, ~10 ml, and is a bit more cumbersome. In either case, solvent and reagent requirements relative to titration are substantially reduced as are the time and cost for analysis.
TLT: What is the basic principle of the FTIR AN method? You also indicate that one can differentiate between strong and weak acids.
van de Voort: The FTIR AN method basically parallels the ASTM titrimetric method. Here, however, it is the reaction of a spectrally active base, sodium phenolate, prepared in a diluent to facilitate reaction with the acids in the oil, resulting in the Na-phenolate absorbance at 1589 cm
-1, dropping proportionately, a classic stoichiometric reaction. For calibration, pure oleic acid is used as the standard, but in this version of the method, the standard is split into two portions, with reagent-diluent being added to one part and plain diluent added to the other (
see Figure 3). The diluent-only treated standard of the pair is used as the background spectrum (I
O) and the reagent-diluent treated standard as the sample spectrum (I). From the ratioed spectra (I/I
O), a differential absorbance spectrum is generated (ΔAbs = -Log T = I/I
O), which has two distinct bands that change as a function of concentration. A negative growing band of unreacted oleic acid in the diluent treated half and a positive decreasing band due to the Na-phenolate signal as it reacts with oleic acid; all of the other common spectral features of the oil matrix are ratioed out.
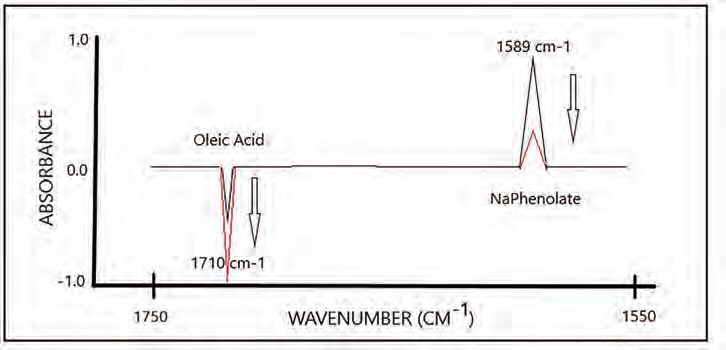 Figure 3. Simulated split-paired sample differential calibration spectra, illustrating the ability to determine total acidity (AN) as well as weak COOH acidity, the difference being acids stronger than COOH.
Viset:
Figure 3. Simulated split-paired sample differential calibration spectra, illustrating the ability to determine total acidity (AN) as well as weak COOH acidity, the difference being acids stronger than COOH.
Viset: As indicated, one adds the alkali, neutralizing all the acids, weak and strong, but a signal specific to weak acids also is there in the spectrum. If you calibrate by splitting the standards first, you obtain an additional calibration information, allowing one to specifically measure the weak acids in in-use oils.
TLT: What is the relevance differentiating between strong and weak acids?
van de Voort: It might be quite relevant. For example, regarding the September TLT Lubrication Fundamentals article titled Engine oil analysis, the article questioned whether one can continue relying on traditional AN and BN measurements. It noted that FTIR CM nitration and oxidation measures would become more relevant as measures indicative of the types of acidity accumulating in in-use oils. The new FTIR AN method we have developed has this ability and can address this issue
quantitatively rather than qualitatively.
TLT: What is the status of the manual FTIR AN method now?
Viset: We have submitted a detailed scientific paper to ASTM International, originally to be presented at the Symposium Highlighting Standard Guides and Practices that Support the Lubricant Condition Monitoring Industry in December 2020 in Texas, but pandemic delayed. I am already using it for gas and hydro turbine oil, DIN 51524 antiwear hydraulic oil and PAO screw compressor oil, and the results make sense. I plan on using it for ISO 320 PAO gear oil, where an annoying ester additive makes the conventional FTIR OX test useless, but the ester is not in play with reagent FTIR. My client only cares about the health of their oil and equipment, and the fact that the method is not ASTM approved is not relevant. The results correlate, and the reagent FTIR method is more precise than the chemometric-based CM systems.
van de Voort: Over the years, I have been urging ASTM International to consider looking into FTIR stoichiometric AN (and BN) determination as an alternative to titration. This has not gone far, in part because automated methods require professional implementation, specialized software and dedicated turnkey FTIR systems. It also requires a cohort of active users, without which they are difficult to standardize and compare on an interlaboratory basis.
Craig Winterfield, vice president at Fluid Life, carried out a structured evaluation and validation of the methodology and published the results indicative of the performance one can achieve. Conventional FTIR CM took years and extensive effort to metamorphose from its JOAP foundation to ASTM E2412 and its component measures. Now that a manual, generic AN protocol has been developed, which is spectrometer-software independent, anyone can quickly implement and assess the method and generate results with a modicum of computing skills or guidance. Although manual, it is still pretty fast as one can analyze up to 20 samples/h. SpectraGryph is free for trial and developmental use, so there is little to hold anyone back from trying it out if the methodology interests them.
TLT: Generic FTIR AN sounds good, but is there not a downside?
van de Voort: Yes and no, as everything depends on the analytical setting. The new method is based on the spectral analysis of two independently prepared samples to obtain a single result, which would be suicidal for a high-volume commercial CM laboratory. In that setting, a single-sample analysis approach is used, with the oleic acid primary calibration supported by a secondary chemometric algorithm to account for spectral variations within the oil class being analyzed (e.g., non-combustion mineral oils). The secondary chemometric component is required as a representative oil is used as the spectral reference for all oils of that class being analyzed. So, in the high-volume CM lab, you need additional chemometric support to get the speed and are, thus, limited to specific groups of oils or classes, while in the manual generic version, one is oil-type independent. You don’t get to go as fast, but you also don’t need chemometrics, as your oil sample also is its own reference, allowing for highly accurate spectral ratioing. It’s the old story of not being able to have your cake and eat it, too.
Viset: There is a lot more upside than downside. The downside is that it isn’t automated and is slower than a robot—which is a showstopper for a commercial lab but not a downside if you have varying oil types to deal with, no more than a handful of samples a day, and your alternative is no result at all, or titration.
TLT: Anything else on the horizon for quantitative FTIR?
Viset: Well, we want to share the method and its benefits via ASTM International and provide a preview to your readers. The next method we want to tackle is moisture. Yes, there is a direct moisture method in conventional direct read FTIR, but it is fraught with problems spectrally, largely because of hydrogen bonding and other OH interferences. One must have a lot of faith in chemometrics and spectral libraries to believe the results. The gold standard for water measurement in diesel and lube oils is Karl Fischer titration (e.g., ASTM D6304), but it is another potentiometric titration with problematic chemicals.
Before retirement, Fred examined an alternate stoichiometric reagent FTIR method for determining water in edible oils to produce a product that is spectrally active and accurately measured. Mineral oils and diesel fuel require a smaller analytical range, as they hold less water, and you can visibly see when they are wet, so higher sensitivity is very important to obtain meaningful results.
Agilent has kindly offered to loan us a small footprint Cary 630 FTIR for evaluation work. It accepts either a conventional transmission cell or the TumblIR® accessory, which we used for the AN work, which makes FTIR of viscous fluids fast and easy so the method can be assessed for either setup. Later, we will look at BN, as we know the chemistry works, but with low sulfur fuels (<10 ppm), there just isn’t as much demand for the test.
van de Voort: I agree with Michael. Water is next; simplicity and generic implementation are the key to getting quantitative FTIR methods assessed and in circulation. The commercial labs are big players, but making AN available to almost anyone should be a positive development if it competes effectively with titration, reduces the analytical cost and the environmental load. Water is being prioritized because it is a corrosion accelerant, and combining the ability to differentiate between strong and weak acids, in conjunction with an accurate moisture determination by FTIR, could prove a potent combination for predictive maintenance in general.
You can reach Frederik van de Voort at Frederik.vandevoort@mcgill.ca.
You can reach Michael Viset at Michal_Viset@hotmail.com.
FOR FURTHER READING
1.
Winterfield, C. and van de Voort, F.R. (2014), “Automated acid and base number determination of mineral-based lubricants by Fourier transform infrared spectroscopy: Commercial laboratory implementation,”
Journal of Laboratory Automation, pp. 1-10. DOI: 10.1177/221 10682 14551825.
2.
Menges, F. SpectraGryph optical spectroscopy software. Available at
www.effemm2.de/spectragryph/.
3.
Winterfield, C. and van de Voort, F.R. (2015), “Quantitative condition monitoring of in-use oils by FTIR spectroscopy,”
Lube Magazine, 127, pp. 24-29.
4.
Tavassoli-Kafrani, M.H., Curtis, J.M. and van de Voort, F.R. (2016), “Stoichiometric determination of moisture in edible oils by mid-FTIR spectroscopy,”
Analytica Chimica Acta, 98, pp. 1-7.