Metal-organic frameworks (MOFs) have the potential to store fuels because they are porous materials with high-surface areas.
A new MOF known as NU-1501 has been synthesized that displays both high gravimetric and volumetric capacities.
Gas adsorption testing showed that NU-1501 demonstrates strong gravimetric deliverable capacities for both methane and hydrogen.
Methane and hydrogen have emerged as potential fuels for alternatively powered natural gas and fuel cell automobiles, respectively. One area of concern is how to effectively and safely store both fuels in these vehicles. Currently, high-pressure compression is required (250 bar for methane and 700 bar for hydrogen), which is expensive and potentially unsafe.
Metal-organic frameworks (MOFs) have emerged as potential solutions for storing both fuels because they are porous materials with high-surface areas in the 2,000 square meter per gram range or higher. In a previous TLT article,
1 the developer of MOFs, Omar Yaghi, indicated they are three-dimensional crystalline structures that contain inorganic units (such as metal oxides) jointed together by organic linkers that are typically carboxylates.
A more recent TLT article discussed the development of a flexible MOF based on cobalt 1,4-benzenedipyrazolate that exhibits strong performance in adsorbing and releasing natural gas, the main component of which is methane.
2 The need for more efficient storage materials exists, particularly because of the challenge in finding one that exhibits both high gravimetric and volumetric capacities.
Omar Farha, associate professor of chemistry at Northwestern University in Evanston, Ill., says, “Storage capacity is not the right metric for evaluating the storage efficacy of a material. The key parameter is deliverable capacity, the amount of stored fuel, such as hydrogen, delivered to the engine during operation.”
The problem is most materials with high gravimetric capacities have low volumetric capacities. In contrast, materials with high volumetric capacities are quite dense and have low gravimetric capacities.
To find a material that maximizes both gravimetric and volumetric capacities, Farha decided to examine potential MOF candidates. He says, “MOFs are a platform technology that can provide a wide variety of options. They are crystalline, so it is easy to determine exactly their composition and can easily be synthesized through self-assembly.”
According to Farha, an ideal storage material will not be too large in size, nor too heavy so that it can meet the needs of the automotive industry. He says, “A new candidate material should work between 100 bar and 5 bar. Deliverable capacity for the 100 bar → 5 bar pressure swing has received exceptional interest because 100 bar is the highest refueling pressure for which all-metal Type I pressure tanks can be safety compliant.”
Researchers have now identified a new MOF material that displays both high gravimetric and volumetric capacities.
The new MOF material, known as NU-1501, is a metal carboxylate derivative synthesized by Farha and his colleagues. An image of the crystal structure of NU-1501 is shown in Figure 2 where the organic linkers and the inorganic units are shown in purple, the aromatic molecule triptycene is shown in grey, and oxygen atoms are in red.
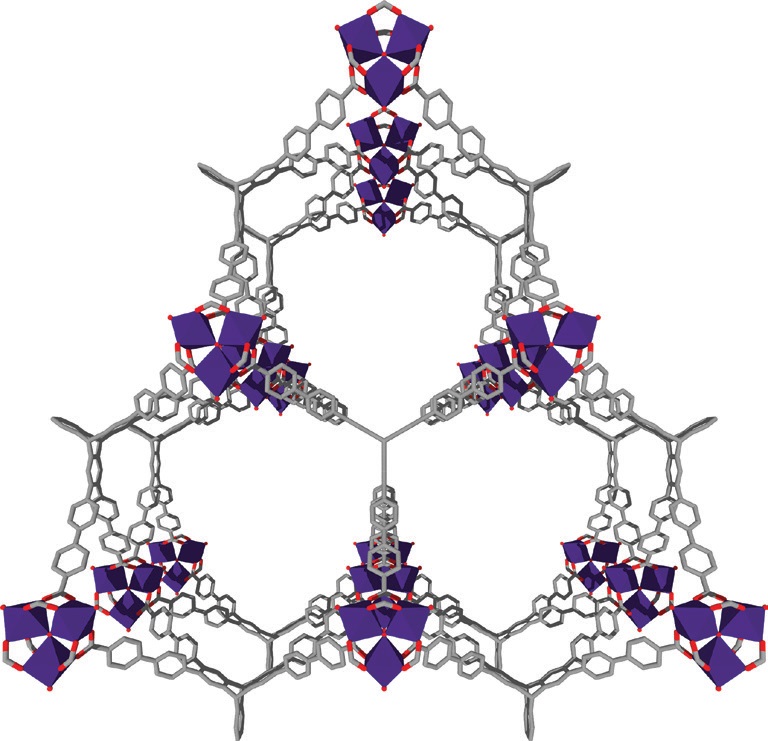 Figure 2. The crystal structure of a MOF material, known as NU-1501, is shown. This MOF displays potential for storing methane and hydrogen. Figure courtesy of Northwestern University.
Figure 2. The crystal structure of a MOF material, known as NU-1501, is shown. This MOF displays potential for storing methane and hydrogen. Figure courtesy of Northwestern University.
Farha says, “We initially evaluated a MOF known as NU-1500, which we made a couple of years ago. The geometric and topological design of this MOF displayed promising characteristics such as high porosity and surface area with a low pore volume and pore size (approximately 1.4 nanometers), good moisture stability and a broad degree of designability.”
The researchers conducted computational modeling and determining that modification of NU-1500 through the use of triptycene produced NU-1501, which displays a high gravimetric area of 7,310 square meters per gram and volumetric area of 2,060 square meters per cubic centimeter. Farha says, “NU-1501 has a 6-c acs topology and has rigid trigonal prismatic triptycene-based organic ligands and aluminum μ3-oxo-centered trinuclear clusters.”
The researchers indicate that a one-gram sample of NU-1501 has a volume of six M&M candies and a surface area that would cover 1.3 football fields. The metal used in NU-1501 can be either aluminum or iron.
Farha says, “Both metals exhibit similar performance, though the aluminum carboxylate connectivity showed better stability. One other important characteristic about NU-1501 is that this MOF only has one dominant pore size of 2.2 nanometers, which is smaller than other MOFs with high gravimetric surface areas.”
Once NU-1501 was prepared, gas adsorption testing was conducted by Farha’s collaborators at NIST. Farha says, “The aluminum version of NU-1501 exhibited a methane gravimetric uptake of 0.66 grams per gram of material and a gravimetric deliverable capacity of 0.60 grams per gram of material under standard temperature and pressure conditions, which both exceed U.S. Department of Energy targets. For hydrogen testing, NU-1501 displayed an outstanding deliverable hydrogen capacity (14% by weight and 46.2 grams/liter) as conditions moved from a temperature and pressure of 77 K and 100 bar to an emptying pressure of 5 bar at a temperature of 160 K. This performance was confirmed multiple times.”
Future work will involve developing NU-1501 as a commercial material for use in storing hydrogen and methane. Two of the challenges are figuring out how to pack the material into a tank so it will take up the least amount of volume and devising a more efficient synthesis procedure. Farha says, “Most MOFs require the preparation of the organic linker separately. We are looking into preparing MOFs with similar performance to NU-1501 in a one-step process.”
Additional information can be found in a recent reference
3 or by contacting Farha at
farha-ofc@northwestern.edu.
REFERENCES
1. Canter, N. (2006), “MOFs: More effective gas adsorbers,” TLT,
62 (4), pp. 12-15.
2. Canter, N. (2016), “More efficient natural gas fuel tank,” TLT,
72 (1), pp. 12-13.
3. Chen, Z., Li, P., Anderson, R., Wang, X., Zhang, X., Robison, L, Redfern, L., Moribe, S., Islamoglu, T., Gualdron, D., Yildirim, T., Stoddard, J. and Farha, O. (2020), “Balancing volumetric and gravimetric uptake in highly porous materials for clean energy,”
Science, 368 (6488), pp. 297-303.