Single-bubble coalescence experiments have been conducted to evaluate how filtration of lubricants impacts the performance of antifoam additives.
The time required for foam bubbles to rupture is correlated to the size distribution of the antifoams used.
Foam stability increases as the micron size of the filter used declines.
Antifoam additives perform the important function of minimizing the generation of foam when a lubricant is in use. Foam, which results from the aggregation of entrained gas bubbles, can cause excessive harm to lubricated machinery. These problems include reduced lubricity, inhibited heat removal, enhanced lubricant oxidation and increased wear to machinery.
In a previous TLT article,
1 a direct correlation was shown to be present between foam stability and evaporation. Through the use of an instrument known as the dynamic fluid-film interferometer (DFI), the researchers devised a new method to study lubricant foaming and discovered a new foam stabilization mechanism in oil-based lubricants.
2 Base oils from API Groups I through IV were evaluated by using the DFI, and foam stability was found to be highest for Group I followed by Group II. The reason is that Group I base oils contain the broad distribution of components (e.g., a large distribution of carbon chains, aromatics, naphthenics, etc.), and differential evaporation of these components leads to greater foam stability. In progressing from Group II to III, the distribution of components steadily declines leading to a reduction in foam stability, with a particular class of Group IV, [i.e., polyalphaolefins (PAO)], being completely derived from synthetic base stocks.
Nonetheless, fully formulated lubricants contain antifoams, which are effective at rupturing bubbles and keeping foam under control for a sustained period. However, these additives are often compromised during lubricant operation. One of the major culprits is filtration, which removes these additives from the oil, thus rendering the lubricant vulnerable to foaming.
Vineeth Chandran Suja, graduate student in the department of chemical engineering at Stanford University in Palo Alto, Calif., and one of the co-authors of the aforementioned study, indicated that past studies evaluating how filtration affects foam generation in lubricants involved the use of bulk foam tests. Suja says, “Lubricant formulators are aware of the deleterious effect of filtration on antifoam removal. Past studies clearly show that the removal of antifoam additives depends upon the filter pore size, the number of filtration cycles, and the initial antifoam volume fraction. However, there is a lack of quantitative understanding of the impact of filtration at the level of single bubbles. Further, there has been no attempt to develop models that can predict the foaming performance of filtered lubricants. Quantifying the impact of filtration and being able to assess the foaming performance of filtered lubricant preemptively will help formulators develop products that will stand the test of filtration.”
The Shell-Stanford team, including Dr. Gerald Fuller, professor of chemical engineering at Stanford, and STLE-member Dr. Abhishek Kar of Shell Global Solutions (US), have now used single-bubble coalescence experiments, with the DFI, to better understand how filtration of lubricants impacts the performance of antifoam additives.
Single-bubble experiments
A typical experiment in this study proceeded as follows. Five to six milliliters of a selected lubricant are charged into the DFI chamber. A bubble is produced with a volume of 1.2 microliters at the tip of a standard 16-gauge capillary, with the size chosen to be closest to the bubble size, having the largest number density in a freshly formed foam. The newly created bubble is moved at a constant velocity of 0.15 millimeters per second towards the lubricant-air interface until the bubble is half a radius above the interface, replicating the dynamics of a free bubble and static foam in a lubricant. During this process, a camera located above the DFI recorded the evolution of the bubble wall thickness. The experiment is completed when the bubble coalesces. Figure 1 shows an interferogram of an antifoam rupturing a bubble.
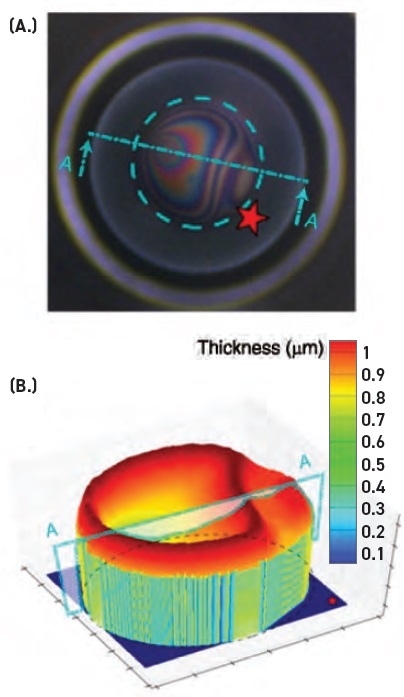 Figure 1. (A.) Interference pattern on a draining bubble, a tenth of a second before an antifoam ruptures a bubble in a lubricant. (B.) A 3D reconstruction of the bubble film thickness from the interference pattern using Fresnel equations. The star shows the orientation of the plot with respect to the interference patterns in panel A. Courtesy of Stanford University and Shell Global Solutions (US).
Figure 1. (A.) Interference pattern on a draining bubble, a tenth of a second before an antifoam ruptures a bubble in a lubricant. (B.) A 3D reconstruction of the bubble film thickness from the interference pattern using Fresnel equations. The star shows the orientation of the plot with respect to the interference patterns in panel A. Courtesy of Stanford University and Shell Global Solutions (US).
The researchers used a commercial gear oil that was formulated with a Group IV base stock. The antifoam additive used was a commercially available siloxane. The formulated lubricants were subjected to a series of experiments with a varying number of filtration cycles, using four different filter pore sizes along with an evaluation of the antifoam volume fraction remaining in the oil after each round of filtration. In all the cases, the fraction of bubbles ruptured before a specific time, hereon referred to as the coalescence time distribution, was measured.
First, the authors established a link between the coalescence time distributions and the antifoam size distributions by verifying Garrett’s hypothesis for the tested antifoam-lubricant systems. Suja says, “Garrett’s hypothesis was proposed in the 1980s and states that the rate-limiting step in bubble coalescence is the time it takes for the film to thin down to the size of the antifoam. In other words, the time required for bubbles to rupture in the lubricant is correlated to the size distribution of the antifoams used.”
For accomplishing this goal, the team used representative antifoams blended in oils where the antifoams were of a known size distribution. Experiments showed that the time required by the bubbles to rupture correlated with this size distribution, thus highlighting the validity of Garett’s hypothesis and establishing a link between coalescence time and antifoam size.
Next, similar bubble coalescence tests were performed on lubricants filtered through a nominal rating of three microns. The researchers found an order of magnitude increase in coalescence time after the first few filtration cycles. Coalescence time did not increase significantly after 10 times the number of filtration cycles. Suja says, “The coalescence time data implied that there is a significant change in the antifoam size distribution during the initial few cycles of filtration as a result of the strong screening of antifoam particles larger than the filter pore size, thus leading to an increase in the time needed to rupture the foam.”
 Foam is known to cause excessive harm to lubricated machinery.
Foam is known to cause excessive harm to lubricated machinery.
As expected, the evaluation of the lubricant filtered using ten, five, three- and one-micron filters led to the conclusion that foam stability increases as the micron size of the filter declines. Suja says, “The coalescence time depends on the duration a bubble film has to drain before it encounters an antifoam particle with a similar size. Hence, the observed results suggest the deactivation of antifoams, possibly through their complete removal or through filtration-flow induced effects such as fragmentation.”
Finally, antifoam volume fraction experiments confirmed that adequate initial antifoam concentration is crucial for the foaming performance of the lubricant. The experiments showed that, in general, the foaming performance is inversely correlated to the initial concentration. Based on this insight, the researchers have also proposed a statistical model that provides a lower bound on the antifoam volume fraction required in a model lubricant for satisfactory foaming performance. This model serves as an initial guide for choosing the adequate initial antifoam volume fraction.
Future work has now extended into looking at water contamination and the role of demulsifiers in minimizing water content in lubricants. Building on their initial work, the researchers will also be looking closely at the mechanism of solutocapillary dynamics in separating water from oil.
Additional information on this research can be found in a recent article
3 or by contacting: Vineeth Chandran Suja at
vinny@stanford.edu , Dr. Fuller at
ggf@stanford.edu or Dr. Kar at
abhishek.kar@shell.com.
REFERENCES
1. Canter, N. (2018), “Foam generation in oil-based lubricants: Evaporation,” TLT,
74 (12), pp. 12-13.
2. Suja, V., Kar, A., Cates, W., Remmert, S., Savage, P. and Fuller, G. (2018), “Evaporation- induced foam stabilization in lubricating oils,” Proceedings of the National Academy of Sciences,
115 (31), pp. 7919-7924.
3. Suja, V., Kar, A., Cates, W., Remmert, S. and Fuller, G. (2020), “Foam stability in filtered lubricants containing antifoams,” Journal of Colloid and Interface Science,
567, pp. 1-9.