ZDDPs have been used in the lubricant industry for 75 years and display multifunctional antiwear and antioxidation characteristics.
A wide variety of ZDDPs are available that function by forming a boundary film on the metal surface to reduce wear and act as a peroxide decomposer to reduce oxidation.
While ZDDP alternatives have been developed, the future for ZDDPs still appears to be promising because this additive type exhibits excellent cost performance.
One of the most widely used additives in automotive and industrial lubricants for the past 75 years has been zinc dialkyldithiophosphates (ZDDPs). Initially developed and patented in 1944 by a representative from the Union Oil Co. of California (
1), ZDDPs have demonstrated multifunctional characteristics that have positioned them to be used prominently in many of the most important lubricants used commercially today.
The first patent identifying ZDDP as a lubricant additive was U.S. Patent 2,261,047 assigned to the Lubri-Zol Corporation (predecessor to The Lubrizol Corporation) and published on October 28, 1941. The inventor of the patent was Peter A. Asseff.
Other organizations also were involved in the development of ZDDPs. U.S. Patent 2,344,392 was assigned to the American Cyanamid Co. and published on March 14, 1944. The inventors were Elmer W. Cook and William D. Thomas, Jr. The Union Oil Company of California was assigned U.S. Patents 2,364,283 and 2,364,284. Both were published on Dec. 5, 1944, and the inventor was Herbert Freuler.
All of these organizations should be credited with assisting in the development of one of the most widely used lubricant additives over the past 75 years.
This additive is prepared in a two-step process where an alcohol is reacted with phosphorus pentasulfide to form a dialkyldithiophosphoric acid intermediate that is then neutralized with zinc oxide (
1).
ZDDPs also have faced regulatory and health and safety issues that have led to the search for potential alternatives. This article provides an update on the current status and future use of ZDDPs and the development of potential alternatives.
Insight on the status of ZDDPs was obtained from representatives at the following organizations:
•
Afton Chemical
•
BASF Corp.
•
Italmatch Chemicals Group
•
King Industries
•
LANXESS Corp.
•
The Lubrizol Corp.
Key applications
Ian Bell, senior R&D director for Afton Chemical in Richmond, Va., says, “ZDDPs are one of the most widely used petroleum additives, providing both wear and oxidation protection. They are ubiquitous in engine oil products and also are featured in other application areas.”
Dr. Annette Loos, application technologist for ZDDPs for LANXESS Corp. in Manheim, Germany, says, “The majority of ZDDP use is in engine oils, but neat metalworking fluids, greases and industrial oils such as hydraulic fluids benefit from ZDDPs as antiwear components and antioxidant synergists. In zinc-containing hydraulic fluids, ZDDPs are the main additive type used at the highest treat rate of all additives needed for this application.”
STLE Past President Dr. Maureen Hunter, technical service manager for King Industries in Norwalk, Conn., considers ZDDPs to be common but extraordinary lubricant additives. She says, “ZDDPs are multifunctional because they provide wear, oxidation and corrosion protection. They are ubiquitous in lubricants and relatively inexpensive. Among the diverse lubricants applications that use ZDDPs are engine oils, rust and oxidation (R&O) oils, hydraulic fluids, turbine oils, compressor oils, gear oils and greases.”
James Vence, technical director for Italmatch Chemicals Group in Cleveland, says, “ZDDPs find their way into industrial and automotive lubricants in a variety of applications from fluids requiring anti-friction and wear properties to improved oxidative stability.”
STLE-member Dr. Eugene Scanlon, technical marketing manager, performance components for BASF Corp. in Tarrytown, N.Y., says, “ZDDPs are used primarily in engine oils, both gasoline and diesel and in some industrial oils with the most prominent one being hydraulic fluids.”
STLE-member Dr. Shubhamita Basu, North American product manager for industrial oils for The Lubrizol Corp. in Wickliffe, Ohio, says, “ZDDPs are extremely good antiwear agents with antioxidation properties and are commonly used in engine oils, antiwear hydraulic fluids and tractor hydraulic fluids.”
Types
As shown in Figure 1, ZDDPs are available in three types known as primary (straight chain), secondary (branched) and alkyl aryl (aromatic) that are based on what alcohol is used. As shown in Figure 1, the performance of these three types as characterized by oxidation inhibition, extreme pressure/antiwear (EP/AW) activity, thermal stability and hydrolytic stability is mostly satisfactory to good with the only poor rating being the hydrolytic stability of the alky aryl type.
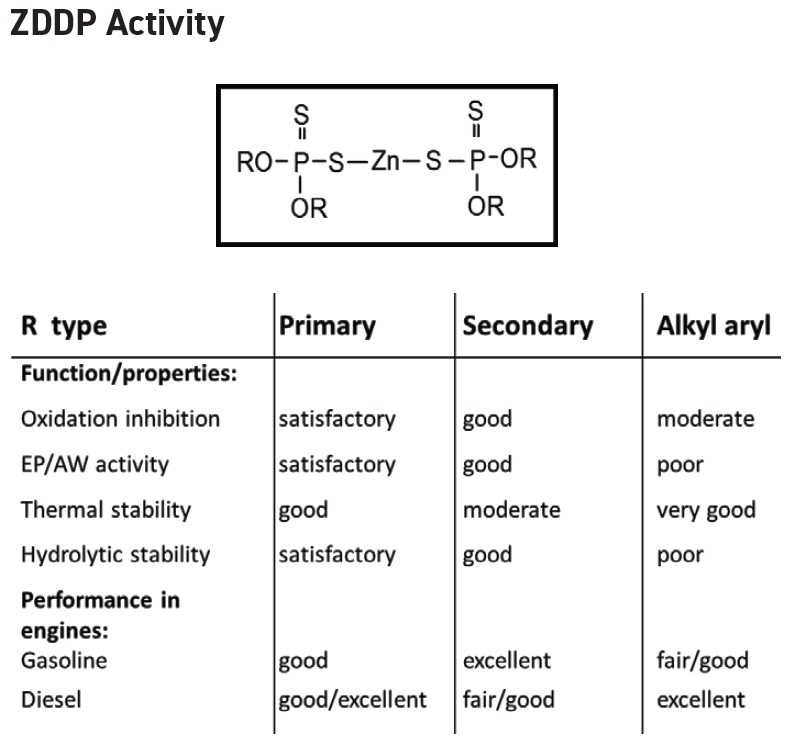 Figure 1. The structure, three types and key properties of ZDDPs are shown. (Figure courtesy of BASF Corp.)
Figure 1. The structure, three types and key properties of ZDDPs are shown. (Figure courtesy of BASF Corp.)
Scanlon says, “The performance of these types of ZDDPs in engine oils for use in gasoline and diesel applications ranges from fair to excellent.”
Vence indicates that alcohols are chosen based on their carbon chain length and degree of branching. He says, “Each ZDDP type has varying degrees of thermal and hydrolytic stability. For example, a multi-purpose, two-primary-alcohol system with different chain lengths would be considered to offer good performance for these two criteria.”
Basu says, “A large variety of ZDDPs are available of which primary and secondary ZDDPs are most common, while some applications use aromatic ZDDPs. Formulators can mix ZDDPs prepared from primary and secondary alcohols to produce a fourth type, known as mixed ZDDPs. Alternatively, mixed ZDDPs can be prepared by mixing primary and secondary alcohols while making the ZDDP.”
Hunter maintains that not all ZDDPs are equal. She says, “Primary ZDDPs have greater thermal stability, less effective antiwear action and less volatile phosphorus, while secondary ZDDPs have lower thermal stability, more effective antiwear action and more volatile phosphorus. The alcohol chain length effects performance in terms of antiwear performance, thermal stability and antirust properties.”
Hunter adds that the rule of thumb for ZDDPs is that the zinc to phosphorus ratio is 1:1 and the sulfur to zinc or phosphorus ratio is 2:1.
Bell indicates that ZDDPs are generally very similar. He says, “The key variance across the specific ZDDPs is the pendant alkyl group that is the result of the alcohols used in their synthesis. The performance function of ZDDPs can be tuned to a specific application by careful selection of the right alcohol moiety: decomposition temperature, wear protection, impact on friction, volatility of the phosphorus species (as measured in engine oils by the Sequence IIIHB test) and oxidation. Various options are open to the formulator: shorter chain alcohols to longer chain alcohols, primary to secondary alcohols or aryl alcohols.”
Dr. Steffen Sandhoefner, senior application technologist for LANXESS Corp., agrees that the nature of the carbon chains have a significant influence on ZDDP performance. “One difference in performance among the various ZDDP types is the binding mode either for primary or secondary alkyl groups and for aromatic groups,” he says. “Alkyl chain length is the second important characteristic that can adjust the performance of ZDDPs to the specific needs of the application. As the alkyl chain length increases, the ZDDP becomes more stable (thermally and hydrolytically) but less reactive. A third important characteristic is the balance between neutral and basic ZDDP salts. Alkalinity has a significant influence on performance and stability.”
Functions and mechanisms
Vence says, “ZDDPs have the ability to mate with the metal surface to provide a sacrificial layer (boundary film) that anticipates the temporary welding of asperities and shearing of the two metal surfaces, thus reducing wear. This additive type imparts antiwear through the build-up of sacrificial layers on metal surfaces that are composed of glassy iron and zinc polyphosphate and metal sulfides (
2).”
Bell says, “In general from an antiwear standpoint, ZDDPs decompose under high-temperature/high-pressure conditions to form a tribofilm in engine applications. This is a highly complex pathway, and the resulting tribofilm is impacted by the type and level of other additive species that are present. However, the readiness of a ZDDP to decompose and the characteristics of the tribofilm can be adjusted by the chemical structure of this additive.”
Bell reveals that as the lubricant ages, the structure of the ZDDP will influence the thickness of the tribofilm. Data produced at two aging temperatures is shown in Figure 2 (
3). Depending upon the temperature, the best-performing ZDDP may be branched, straight chain or a combination of the two.
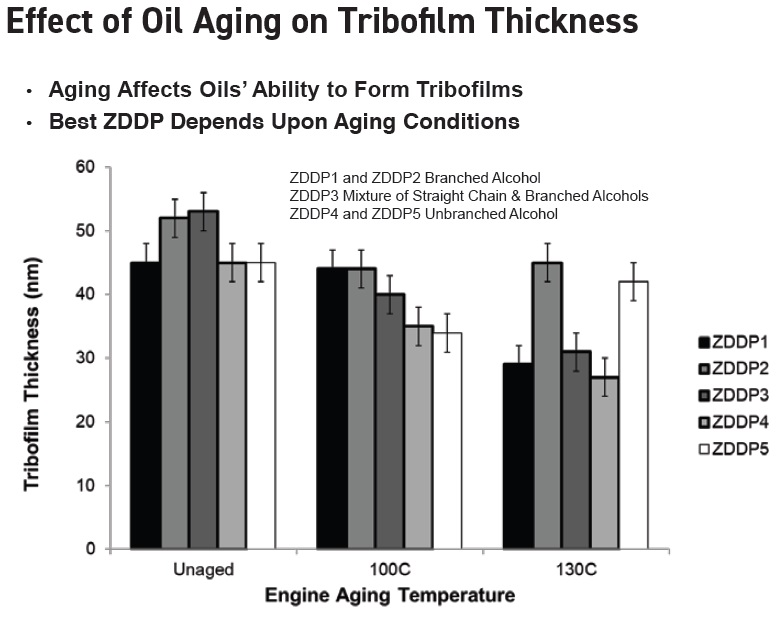 Figure 2. After aging, the thickness of the tribofilm varies by temperature and type of ZDDP. (Figure courtesy of Afton Chemical.)
Figure 2. After aging, the thickness of the tribofilm varies by temperature and type of ZDDP. (Figure courtesy of Afton Chemical.)
Bell continues, “In terms of oxidation, ZDDPs act as peroxide decomposers, cutting into the chain reaction that propagates oxidation in lubricants.”
Scanlon defines the antiwear mechanism of ZDDP as a tribofragmentation. He says, “Usually through heat and/or friction, the additive decomposes and forms a phosphate film on the metal surface (
4). As an antioxidant, ZDDPs are peroxide decomposers, which in the lubricant industry is called a secondary antioxidant. Most peroxide decomposers contain either divalent or tetravalent sulfur or trivalent phosphorus, which reduces the hydroperoxide.”
Hunter says, “ZDDPs function as secondary antioxidants by preventing the split of hydroperoxides into extremely reactive alkoxy and hydroxy radicals. They act by converting hydroperoxides into nonradical, nonreactive, thermally stable products that do not participate in further oxidation of the lubricant. ZDDPs are often used in combination with primary antioxidants to yield synergistic antioxidation properties.”
Interaction
Sandhoefner indicates that ZDDPs can interact positively with other additives and therefore are used in additive packages. He says, “ZDDP-containing additive packages are formulated by using as many positive combinations as possible through avoiding incompatibilities among specific additives. This can be challenging because modern packages often end up with a combination of 10 or more raw materials. Empirical knowledge of synergistic interactions is the key to preparing stable and functional packages.”
ZDDPs have been found to be synergistic with some friction modifiers according to Scanlon. He says, “There are some published examples where certain friction modifiers require a phosphate film before friction reduction can occur (
5).”
Bell says, “Lubricants, especially engine oils, are complex systems containing many additive species. It is therefore critical to understand the various interactions in bulk as the additives function. One such interaction is with molybdenum-based friction modifiers (Moly FMs). ZDDPs and Moly FMs do not form discrete surface layers in the engine. The effectiveness of a Moly FM is highly impacted by the ZDDP.
“In a similar manner, the calcium and magnesium cations used in overbased engine oil detergents interact with ZDDPs,” Bell adds. “Both calcium and magnesium can be incorporated into the ZDDP-derived surface layer, impacting the friction of that film. Magnesium has been found to be more detrimental than calcium.”
Figure 3 illustrates how tribofilm thickness changed depending on which of seven friction modifiers used at the same treat was combined with the same ZDDP (
6). Six of the friction modifiers produced a significant reduction in tribofilm thickness.
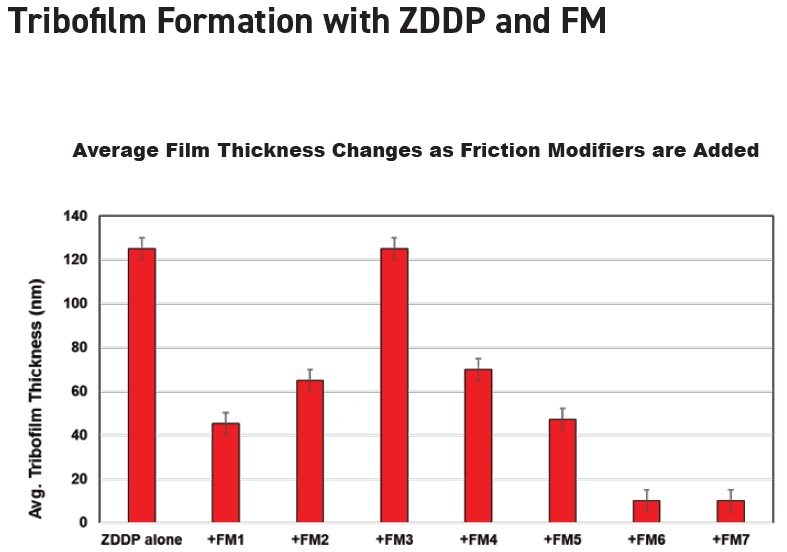 Figure 3. Different average film thickness values are seen when the same ZDDP interacts with seven friction modifiers. (Figure courtesy of Afton Chemical.)
Figure 3. Different average film thickness values are seen when the same ZDDP interacts with seven friction modifiers. (Figure courtesy of Afton Chemical.)
Vence points out that ZDDP is synergistic with a number of components used in formulating lubricants. Two examples are sulfurized additives and borate esters. He says, “When ZDDP is combined with sulfurized products at specific ratios, EP performance can be optimized higher than if both products are used separately. The correct ratio is important because both additive types compete for the metal surface. ZDDP also is a great base for controlling copper corrosion and allows for the use of higher concentrations of active sulfur.”
Vence claims that the synergism between ZDDPs and borate esters in complexing a simple lithium soap-based grease is often overlooked. “It is well known that borate esters can increase the dropping point of a simple lithium soap-based grease to about 220 C when just added alone,” he says. “When ZDDP is present in the formulation, the dropping point can exceed 300 C.”
Negative characteristics
From an engine oil standpoint, Bell indicates that the negative aspects of ZDDPs are associated with their chemical and physical impacts on vehicle-aftertreatment compatibility. “The associated phosphorus can poison catalysts, and the non-combustible metal can contribute to ash build up in exhaust filters,” he says.
In response, the lubricant industry has taken measures to minimize these negative characteristics. Bell adds: “The lubricant industry has reduced the application of ZDDPs via phosphorus limits in OEM and industry specifications. As far back as 1998, the first purposeful reductions in phosphorus levels were implemented, and this process has continued in passenger car motor oil (PCMO) and heavy-duty diesel (HDD) applications.”
One other factor is the concern faced in reducing ash levels. Bell says, “This has put pressure on ZDDP use but only at a ‘total ash’ level—leaving the formulator the opportunity to choose where they use their ‘ration’ of ash.”
Scanlon provides the following three negatives concerning the use of ZDDPs.
1.
Certain ZDDPs are too volatile and can cause issues with exhaust aftertreatment devices in automotive applications.
2.
ZDDP contains zinc that generates ash upon combustion. Most modern diesel engines have exhaust aftertreatment particulate traps, which can be harmed from ash. The trap becomes plugged with ash and works less efficiently.
3.
ZDDP is aquatically toxic. This can become an issue in certain environmentally sensitive applications, i.e., forestry, marine, etc.
Hunter indicates that ZDDPs can have a significant negative impact on the demulsibility performance of an oil. She says, “Figure 4 shows the demulsibility performance to three milliliters of emulsion or less. The base oil alone has excellent demulsibility, requiring five minutes to separate. The addition of 0.6% ZDDP destroys the demulsibility performance; no separation is observed even after 60 minutes. However, some additives including metal alkyl naphthalene sulfonates (DNN) can overcome the adverse effect of the ZDDP, greatly improving the demulsibility.”
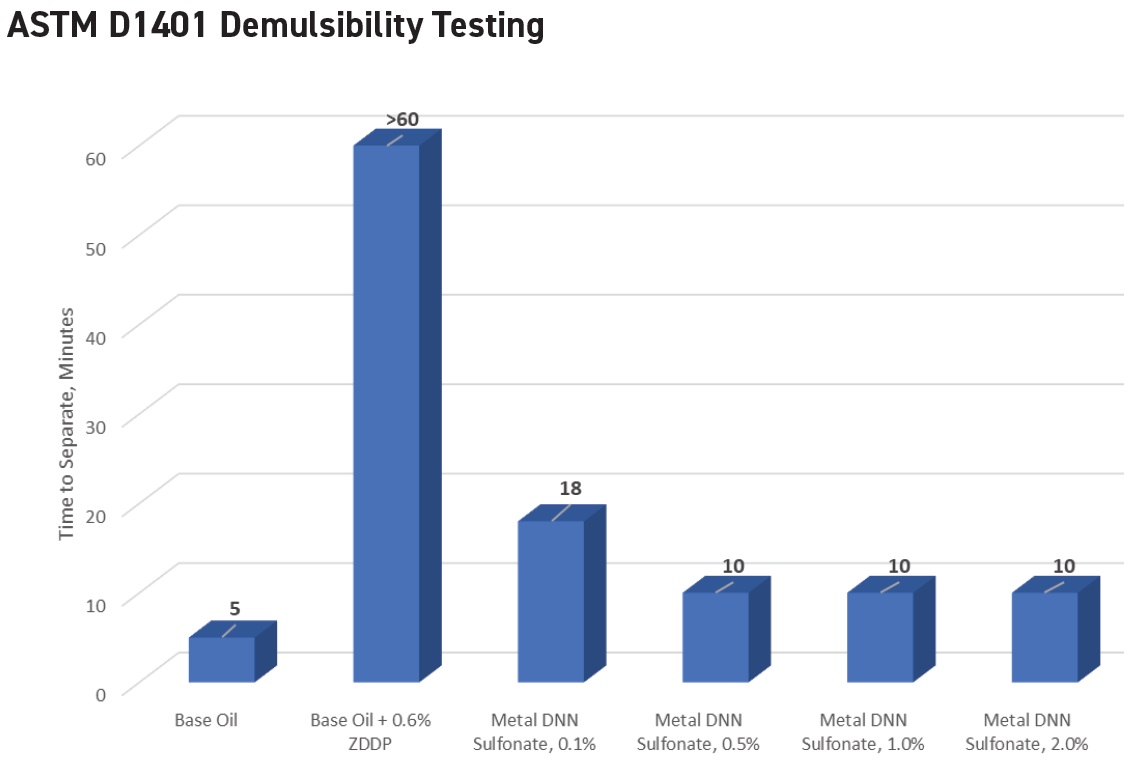 Figure 4. The tendency of ZDDPs to inhibit demulsibility can be reduced through additives such as metal alkyl naphthalene sulfonates. (Figure courtesy of King Industries.)
Figure 4. The tendency of ZDDPs to inhibit demulsibility can be reduced through additives such as metal alkyl naphthalene sulfonates. (Figure courtesy of King Industries.)
Vence believes that the negatives for ZDDPs are few and far. He says, “If the concentration of a ZDDP is too high in a specific formulation, the effectiveness of EP additives can be reduced due to competition on the metal surface. This puts the formulator in a position to do a balancing act to find the correct ratio of ZDDP:EP to maximize performance.”
Vence continues, “Regulation of ZDDP could limit its use in the future. Whether there will be a limit on the types or concentration of ZDDP in specific lubricants is a question mark.”
Loos says, “ZDDPs can negatively react with certain additives to form zinc salts that can form precipitates or even generate sludge formation. Lubricant stability in ZDDP containing formulations is only a given as long as the oil remains basic because ZDDPs become unstable under acidic conditions. A third concern with using ZDDPs is regulatory. This additive is not suitable for environmentally friendly lubricant formulations.”
Basu says, “Some ZDDPs have poor thermal and hydrolytic stability, so the lubricant formulator must keep this in mind in choosing the right one for a specific application.”
Alternatives
Scanlon states that the lubricant industry has developed a number of ZDDP alternatives. He says, “Phosphates, dithiophosphates and phosphorothionates have been commercialized that are prepared with other metals or are ashless (metal free). Organometallic derivatives also have been produced that contain molybdenum, titanium and tungsten.”
Loos says, “If treat rate and AW performance only are taken into consideration, there are phosphorus containing AW additives available that can be used at lower dosage levels than ZDDPs to gain the same performance level. But often these products (e.g., amine neutralized phosphate esters) show antagonistic effects on antioxidant performance and also often cannot be combined with metal containing additives. The cost of these ZDDP alternatives also is usually higher. For these reasons, ZDDPs continue to be so widely used today because they display extremely good multifunctional properties (provide AW and antioxidant performance and show hardly any antagonism with other additive types) combined with an extremely attractive price level.”
Vence believes that it is challenging to find a single additive to directly replace ZDDPs. “ZDDPs are multifunctional additives that provide antiwear protection but also act as antioxidants and corrosion inhibitors,” he says. “They work synergistically with EP components such as sulfurized olefins and triglycerides and various corrosion inhibitors. It is not possible to find a single component that would provide all these characteristics especially at a comparable treat rate and be price competitive.”
Vence feels that the best strategy for replacing ZDDPs is to find an optimum mixture of ashless components to meet the desired requirements of the finished product, rather than just replacing a specific ZDDP with a different AW component. He says, “Careful balancing of EP-AW components, antioxidants and yellow passivators lead to developing additives that can have the same performance characteristics as ZDDPs. This approach is particularly important in the two main industrial lubricant applications where ZDDPs are widely used, hydraulic oils and greases.”
Table 1 shows that the performance of an ashless antiwear hydraulic fluid is comparable to a ZDDP containing hydraulic fluid. Vence says, “Our approach in developing a ZDDP alternative hydraulic fluid was to combine different antiwear chemistries that meet the same performance criteria. The hydraulic fluids were prepared using an ISO 46 Group II base oil.”
Table 1. An ashless hydraulic fluid that does not contain ZDDP displays comparable performance to a ZDDP-based hydraulic fluid. Both fluids were prepared with an ISO VG 46 Group II base oil. (Table courtesy of Italmatch Chemicals Group.)
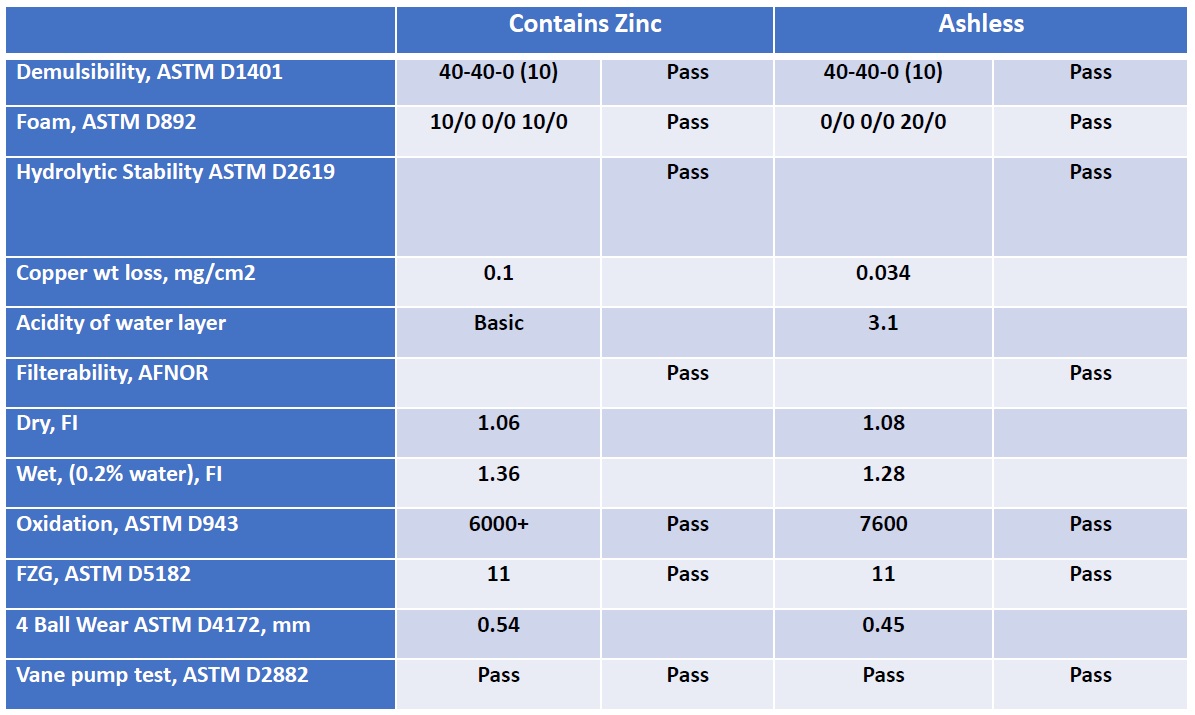
Vence continues, “In many instances, ashless hydraulic additives have an advantage, as they may be used in multiple applications—such as compressor, light gear, biodegradable and biofriendly lubricants where a zinc-containing product is not desirable. Cost remains the main drawback in selecting ashless additives versus choosing ZDDPs.”
Vence expressed concern with mixing ashless with ZDDP-based hydraulic fluids. He says, “The two fluids may be incompatible because acidic components often formulated into ashless hydraulic fluids may have negative interactions with basic components used in ZDDP-based hydraulic fluids such as overbased metal sulfonates. Cross-contamination of the two fluids may result in loss of performance, especially filterability. When switching from a ZDDP-based to an ashless hydraulic fluid, thorough flushing of equipment may be necessary.”
In Table 2, identical treat rates of an ashless dialkyldithiophosphate and a zinc dialkyldithiophosphate are mixed with a blend of a yellow metal deactivator, an aminic antioxidant and a phenolic antioxidant in an ISO VG 46 Group I base oil and evaluated for wear, EP, oxidation resistance, ferrous and copper corrosion, demulsibility and filtration. Hunter says, “The results indicate that an ashless alternative can produce comparable results to a ZDDP-based lubricant.”
Table 2. Comparable performance was found when an ashless dialkyldithiophosphate and a ZDDP were mixed with the same additive blend. (Table courtesy of King Industries.)
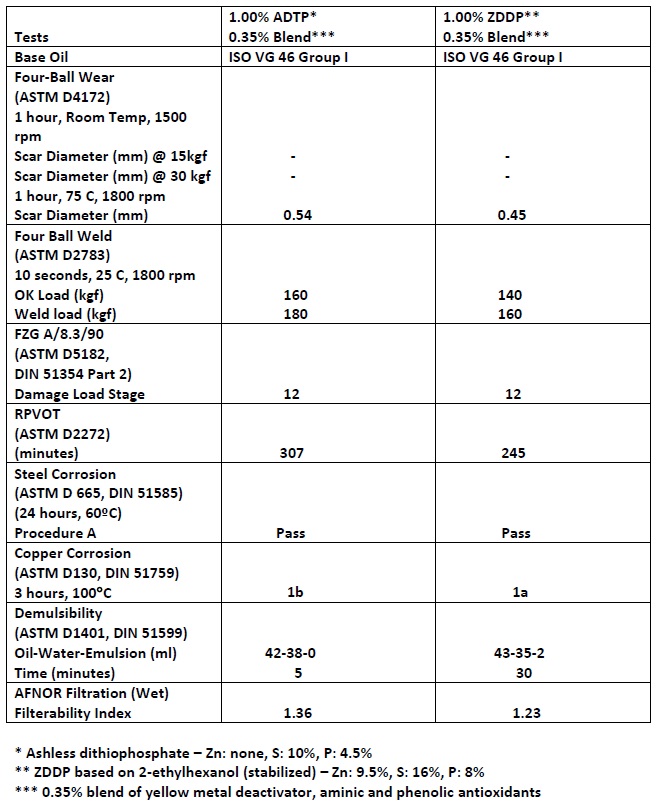
Hunter noted that formulators should take into consideration that ZDDPs are more thermally stable and more polar than ashless dialkyldithiophosphates.
Basu indicates that ZDDP alternatives are used in hydraulic fluids. She says, “Hydraulic applications that are operating in environmentally sensitive areas are ones where zinc-free formulations are preferred—for example, golf courses, waterways, off-shore applications and forestry. In most of these cases, zinc-free formulations meet the requirements of zinc-containing formulations such as DIN 51524, ISO 11158, ASTM D6158, Eaton E-FDGN-TB002-E and Denison HF-0. If there is a regulatory driver where a lubricant must meet a standard such as the EU Ecolabel, then a zinc-free formulation must be used to meet this requirement.”
Sandhoefner says, “Most ZDDP alternative products are metal free and based on phosphorus technology. They often exhibit slightly acidic characteristics, which means that replacing them in metal-containing formulations can lead to a risk for the formation of precipitates. For this reason, nonionic, phosphate ester additives are one option that has only a slight risk for the generation of sludge or other precipitates.”
Scanlon indicates that a number of ZDDP alternatives are available and are used in industrial and automotive lubricants. He says, “Ashless organic dialkyldithiophosphates are used quite often in industrial applications with similar or even better antiwear performance. Molybdenum dithiocarbamate is finding increasing use in engine oils and works as both an antiwear and friction-reducing additive. There also are organo-titanium and organo-tungsten compounds that have been used in some commercially available lubricants.”
Scanlon feels that ZDDP alternatives are mostly used in environmentally sensitive applications that cannot tolerate metals. He says, “They have enjoyed many decades of successful use.”
One of the concerns with working with ZDDP alternatives in formulating lubricants is filter plugging. Scanlon says, “Some ashless additives can form complexes with other metal-containing additive such as those that contain calcium, barium or sodium. The result is the new complex can become physically trapped in the filter leading to filtration problems and additive depletion.”
Bell believes that ZDDP alternatives are usually seen as performance boosts for AW. He says, “They can be from several chemical families and behave differently than ZDDP when interacting with other additives used in lubricant formulations. Additives will always interact, and it is the subtle management of this that is the skill of the industry’s formulators. In switching to a ZDDP alternative, the formulator will trade one interaction for another, which may help or hinder depending upon the additives used.”
Backward compatibility
The movement to lower levels of ZDDPs in engine oils has led to concern about whether today’s lubricants will be able to protect older engines from wear and be backward compatible. Dr. Ewan Delbridge, global manager, consumer engine lubricants for the Lubrizol Corp., says, “Each passenger car specification upgrade is just that, an upgrade. The new category supersedes the old and is backward compatible.
This means a GF-6 credentialed oil will protect engines designed for GF-5, 4, 3, 2 and 1 oils. In fact, older engines benefit from newer oils because all performance dimensions have increased with time and in some cases, new performance dimensions have been introduced (e.g., GF-6 has a chain wear and a low-speed preignition [LSPI] engine test—neither of which were present in GF-5 or earlier categories). To ensure backward compatibility, all global passenger car specifications have tests that measure wear.”
Bell says, “This issue is a fair concern. However, the OEMs and the lubricant industry work very hard to ensure that the vehicles and the fluids available in the market are suitable for use. There are cases where certain forward-looking fluids are restricted in use through the definition of a new specification (API FA-4, ILSAC GF6B), and this requires consumer awareness to ensure that the correct fluid is used. Perhaps we will start to see greater diversification of these modern fluids and the start of a split from the retrospective needs of heritage vehicles. Nevertheless, modern fluids are highly capable of protecting current and older engines.”
The future
Bell believes that the future of ZDDPs will be managed because the automotive industry is headed toward dealing with challenges of increased fuel economy, lower emissions and advanced aftertreatment devices. He says, “Directionally, we would see ZDDP use being reduced in next-generation applications (timeline to be determined) driven by reduced ash and by the need for more fuel economy credits. This will, as ever, be led by PCMO, but HDD will be following along closely. Perhaps the user need for durability assurances in HDD will result in ZDDPs seeing prolonged use as compared to PCMO.”
Basu says, “Change in ZDDP use will be application dependent. ZDDP is a very cost-effective antiwear additive. Growth prospects of ashless chemistry could be limited unless driven by regulation.”
Sandhoefner feels it is difficult to make reliable predictions. “Some years ago, a former colleague, now retired, indicated that the first research project he worked on when he came into the lubricant industry was to find a replacement for ZDDPs. Now, decades later, ZDDPs continue to be the most important AW additive class in the market and are used in much larger quantities than any other AW additive.”
Vence contrasted how ZDDP use will be influenced by changes in automotive versus industrial lubricant applications. “As the biggest volume of ZDDP is used by the automotive industry, changes in this industry will drive changes in the ZDDP market,” he says.” “New emission regulations and improvements in engine design resulted in significant reduction in the amount of ZDDP. Moving from internal combustion engines to electric vehicles will most likely impact the demand and composition of automotive lubricants in the future.”
Vence adds: “In industrial oil markets, increasing requests are being made for ashless additives to replace ZDDPs in hydraulic and grease applications. But we do not see this becoming a significant direction unless regulations for using ZDDPs change.”
ZDDPs will continue to be used widely in the future in automotive and industrial lubricant applications. Change in their use will be dictated by future regulations.
REFERENCES
1.
McDonald, R. (2017), “Zinc Dithiophosphates,” in
Lubricant Additives, Chemistry and Applications, 3rd Edition, Rudnick, L., editor, CRC Press, Boca Raton, Fla., p. 37.
2.
Spikes, H. (2004), “The History and Mechanisms of ZDDP,”
Tribology Letters,
17 (3), pp. 469-489.
3.
Devlin, M., Dovark, T., Garelick, K., Guevremont, J., Guinther, G., Hux, K., Loper, J. and Sheets, R. (2010) “Effect of Fluid Aging on ZDDP Tribofilm Formation and Tribofilm Properties,” Presented at 2010 China (Changchun) International Automotive Forum & SAE-China Congress.
4.
Fujita, H., Glovanea, R. and Spikes, H. (2005), “Study of Zinc Dialkyldithiophosphate Antiwear Film Formation and Removal Processes, Part 1: Experimental,”
Tribology Transactions,
48 (4), pp. 558-566.
5.
Miklozic, K., Forbus, T. and Spikes, H. (2007), “Performance of Friction Modifiers on ZDDP-Generated Surfaces,”
Tribology Transactions,”
50 (3), pp. 328-335.
6.
Guevremont, J., Garelick, K., Loper, J., Lagona, J., Sheets, R., Hux, K. and Devlin, M. (2010), “Influence of Friction Modifiers on Boundary Film Formation Properties,” Presented at the 65th STLE Annual Meeting and Exhibition in Las Vegas, Nev., May 2010.