Lithium cobalt oxide has emerged as the most popular cathode material used in lithium-ion batteries but has several negative characteristics.
A new cathode material based on vanadium disulfide exhibits a faster charge/discharge cycle than a battery using lithium cobalt oxide.
A thin titanium disulfide coating stabilized the vanadium disulfide cathode.
Lithium-ion batteries continue to be the subject of an intensive research effort as they are considered the most popular option at this time to power electric vehicles. But this battery type has encountered operational difficulties including flammability.
Despite the focus on lithium-ion batteries, researchers also have evaluated other types. For example, a previous TLT article (
1) discussed the development of a new type of magnesium battery. In particular, a newly designed cathode based on an organic polymer was discussed that displayed the most stable cycling performance for a non-aqueous magnesium battery. The organic polymers used were a polymeric quinone or a conjugated redox polymer. One other attractive characteristic of this new battery design was that a lean electrolyte design was used.
The most popular cathode material used in a lithium-ion battery is lithium cobalt oxide. Nikhil Koratkar, professor of mechanical, aerospace and nuclear engineering at Rensselaer Polytechnic Institute in Troy, N.Y., says, “One of the key factors in maximizing the performance of a lithium-ion battery is enabling lithium ions to more rapidly move between the anode and the cathode. Lithium cobalt oxide has been satisfactory, but we believe further performance improvements can be made.”
Koratkar indicates that there are several concerns about continuing to work with cobalt. He says, “Cobalt is a heavy element that adds weight to a battery. In addition, the future availability of cobalt is a concern since it is only available through mining in one specific region. Cobalt also is a toxic material that has been found to be hazardous to humans.”
One option to be used in replacing cobalt is vanadium disulfide. Koratkar says, “This material is a transition metal dichalcogenide that is electrically conductive, lighter in weight than cobalt and environmentally safe. Vanadium disulfide has the potential to further increase energy density because of its lower weight and its high conductivity improves the fast charging capability of the lithium-ion battery.”
The problem in using vanadium disulfide has been instability. Koratkar says, “Lithium-ion batteries using vanadium disulfide as a cathode have suffered from poor stability that leads to low cycle life. During charge/discharge cycles, the planar structure of vanadium disulfide expands and breaks apart by overcoming the weak van der Waals forces holding the material together. Lattice distortion results as the atoms are pushed apart and pulled together creating the instability. The source of the problem is specifically the vanadium and not the sulfur atoms.”
The researchers characterized this phenomenon as the Peierls Distortion.
A new approach has now been developed to stabilize vanadium disulfide so that it can be used as a cathode material.
Titanium disulfide
Koratkar and his colleagues were able to stabilize vanadium disulfides by applying a thin layer of titanium disulfide. He says, “We found that titanium disulfide is not vulnerable to the Peierls Distortion in the same manner as vanadium disulfide when used as a cathode in a lithium-ion battery. The main advantage in using a titanium disulfide layer is that this material does not peel off and is mechanically stable over at least 400-500 battery cycles.”
The researchers were able to achieve this result using a titanium disulfide coating that is approximately 2.5 nanometers thick. Titanium disulfide sheets were deposited on vanadium disulfide platelets through the use of atomic layer deposition. Figure 3 is a top-view scanning electron microscope image showing vanadium disulfide crystals in the top two images and titanium disulfide coated flakes in the bottom two images. These images show the compatibility of titanium disulfide with vanadium disulfide.
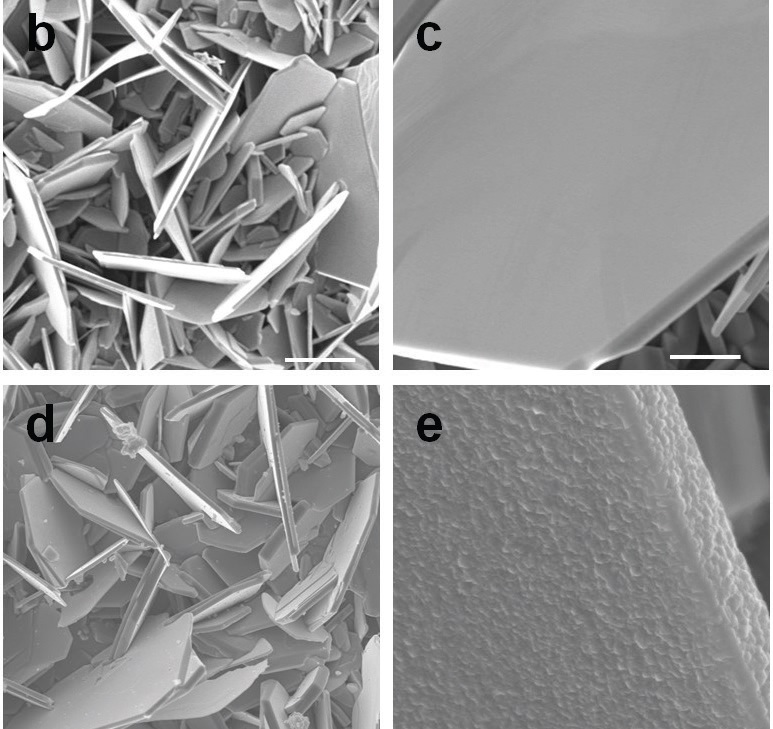 Figure 3. Vandium disulfide’s use as a cathode in lithium-ion batteries was enhanced by a titanium disulfide coating. The top two scanning electron microscope images showing vanadium disulfide crystals, and the bottom two images showing the titanium disulfide coating illustrate the compatibility of the two materials. (Figure courtesy of Rensselaer Polytechnic Institute.)
Figure 3. Vandium disulfide’s use as a cathode in lithium-ion batteries was enhanced by a titanium disulfide coating. The top two scanning electron microscope images showing vanadium disulfide crystals, and the bottom two images showing the titanium disulfide coating illustrate the compatibility of the two materials. (Figure courtesy of Rensselaer Polytechnic Institute.)
Performance testing demonstrated that the biggest benefit for a lithium-ion battery containing the titanium disulfide coated cathode was a more rapid charge/discharge cycle. Koratkar says, “We found that the titanium disulfide coated electrode achieved 2 C-3 C cycling, which means the duration of a cycle is between 20 and 30 minutes. In contrast, a conventional lithium-ion battery with a lithium cobalt oxide cathode exhibited a 0.5 C cycle, which lasts two hours.”
The one weakness of the titanium disulfide coated cathode battery is operating voltage. Koratkar says, “The operating voltage for our newly developed battery is 2 Volts, which is lower than what is seen for a conventional lithium-ion battery (3.5 Volts).”
The stability of the titanium disulfide coated cathode was determined by an optical microscopy study. Koratkar says, “We prepared smaller flakes of vanadium disulfide and titanium disulfide coated vanadium disulfide (20 nanometers in thickness) and observed structural changes during charge/discharge cycles. The vanadium disulfide flake became more transparent and the transparency spread over the flake as the cycling progressed. In contrast, the titanium disulfide coated flake showed no change in transparency indicating better stability.”
Initial evaluation work was done with coin cells and for the future, Koratkar would like to evaluate titanium disulfide coated cathodes in larger battery cells. He adds, “We also would like to increase the scalability of the batteries by building cells with larger mass loadings.”
Additional information can be found in a recent article (
2) or by contacting Koratkar at
koratn@rpi.edu.
REFERENCES
1.
Canter, N. (2019), “New Type of Magnesium Battery,” TLT,
75 (4), pp. 16-17.
2.
Li, L., Yoshimura, A., Sun, C., Wang, T., Chen, Y., Chen, Z., Littlejohn, A., Xiang, Y., Hundekar, P., Bartolucci, S., Shi, J., Shi, Su., Meunier, V., Wang, G. and Koartkar, N. (2019), “Vanadium Disulfide Flakes with Nanolayered Titanium Disulfide Coating as Cathode Materials in Lithium-Ion Batteries,”
Nature Communications,
10, Article Number: 1764.