A newly developed instrument known as a dynamic fluid-film interferometer studies foaming in lubricant base oils.
In studying Group I-V base oils, the researchers found that the base oil with the highest foam stability, Group I, also has the broadest distribution of components.
The more stable foam is due to differential evaporation of the multicomponent base oils.
Foam continues to be a problem that formulators and end-users of lubricants must overcome. Air is an inferior lubricant, and its excessive presence due to foam is harmful in lubricant systems. The presence of foam can cause operational difficulties such as reducing lubricity, preventing heat removal and increasing wear.
One lubricant application where foam continues to be problematic is metalworking fluids. In a previous TLT article, the causes of foam in metalworking fluids were discussed (
1). For water-based metalworking fluids, the emulsifier(s) which operates at the interface between air and the metalworking fluid can be one of the main sources for foam generation.
In a recent publication in PNAS (
2), a collaborative team from Stanford and Shell (Formulations Division) describe the physical mechanisms responsible for foam stabilization in different grades of lubricant base oils. Graduate student Vineeth Chandran Suja in the chemical engineering department at Stanford University in Palo Alto, Calif., working with STLE-member and scientist Dr. Abhishek Kar of Shell Global Solutions US in Houston, developed a strategy for examining the coalescence dynamics of single bubbles in lubricant systems. The focus was mostly on the mechanism behind bubble stability in base oils used in these lubricants and further classifying these systems based on their ability to stabilize foams.
Current procedures for evaluating foam rely on the analysis of bulk foam, Suja says. “Test procedures such as ASTM D892 (Standard Test Method for Foaming Characteristics of Lubricating Oils) only provide information on the stability and density of the aggregate foam,” says Suja. “However, the method does not reveal insights on the mechanism behind foam stability, which can be used to formulate products that are resistant to foaming.”
Single-film experiments that use techniques such as the Scheludko cell can directly probe the mechanisms for why foam is produced. Suja says, “These foam tests still have limitations due to their inability to use full bubbles and simulate bubble coalescence at flat liquid-air interfaces.”
Suja pointed out that current thinking for how foam is generated in base oils is focused on viscosity. He says, “There is a view that the high viscosity of some oils will stabilize foam. But other sources of foam include the build up of contaminants that can stabilize foam.”
For contaminant-free lubricant base oils, the viscosity theory still does not appear to completely explain foaming because past studies evaluating base oils from the Group I, II, III and IV categories with identical viscosities led to the generation of different levels of foam when the same test procedure is used.
Further work to determine the mechanism for foam generation in lubricant base oils has now been conducted using a newly developed experimental technique.
Dynamic fluid-film interferometer
Suja and his colleagues, including Dr. Gerald Fuller, professor of chemical engineering at Stanford University, in collaboration with Dr. Kar and his colleagues at Shell, used an instrument developed at the Fuller Lab known as the dynamic fluid-film interferometer (DFI) to study foaming in lubricant base oils. Using this device, they have uncovered a new mechanism for foaming in oil-based lubricants. He says, “We create a bubble with a size of approximately 1.5 millimeters on a capillary and then measure the time it takes (known as the coalescence time) for the bubble to rupture at the lubricant-air interface. As part of this process, the internal bubble pressure is measured to accurately determine the coalescence time. A camera at the top of the instrument visualizes the spatio-temporal evolution of the bubble wall.”
The DFI simulates the movement of a freely rising bubble made to come to rest by balancing buoyancy with surface tension. Subsequently, the evolution of the bubble wall thickness is visualized as radiant colors generated through thin film interferometry. Suja says, “We found that a darker color is associated with a lower degree of foam and correlates to a thinner bubble.”
The researchers measured the foaming tendency of Group I, II, III, IV and V base oils at 20 C. The viscosities of these base oils at 40 C are comparable and range from 18.2 cSt to 35 cSt. The Group V base oil, which is generally a catchall for all sorts of base oils that cannot be classified into Groups I-IV, is a silicone oil.
Test results indicated that the Group I base oil displayed the highest foam stability followed by Group II, III and IV. This finding also was seen from bulk foam experiments run on the same base oils.
The researchers believe the differences in performance are directly related to the broadness of the distribution of components in the base oils. Group I base oils display the highest distribution of components while Group IV base oils exhibit a very small distribution.
Suja says, “We believe that foam stability is due to differential evaporation of multicomponent base oils. As the lighter components evaporate, the surface tension of the resulting oil increases. This creates a gradient in surface tension, resulting in tears-of-wine-like flows that draws additional oil to the top of the bubble leading to a thicker walled bubble that is less likely to burst.”
Interestingly, as seen in Figure 1, this entire bubble stabilization process is very dynamic due to the complex interplay of forces that drain (or destabilize) the bubble and the tears-of-wine like flows that stabilize the bubble. The various stages in the process are highlighted in different panels. The top image shows the process of oil enrichment, which leads to the formation of a dimple (bottom left image) that stabilizes the bubble. Ambient disturbances (bottom right image) eventually destabilize the dimple leading to a washout process returning the bubble to its original appearance in the top image. The cycle then repeats.
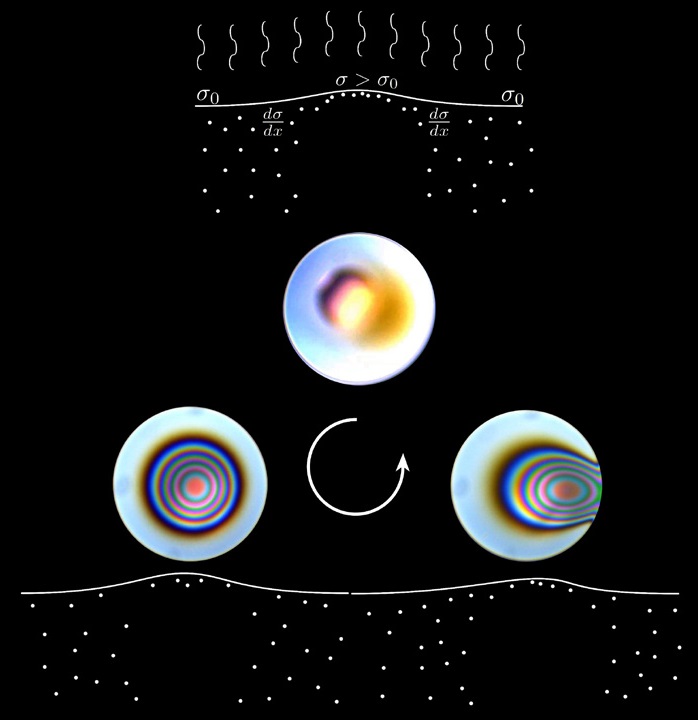 Figure 1. The bubble stabilization process is illustrated with the top image showing oil enrichment that leads to dimple formation (bottom left image). Ambient disturbances (bottom right image) destabilize the dimple completing the cycle as the bubble reverts back to its original appearance (top image). (Figure courtesy of Stanford University and Shell Global Solutions US.)
Figure 1. The bubble stabilization process is illustrated with the top image showing oil enrichment that leads to dimple formation (bottom left image). Ambient disturbances (bottom right image) destabilize the dimple completing the cycle as the bubble reverts back to its original appearance (top image). (Figure courtesy of Stanford University and Shell Global Solutions US.)
A study comparing a pure silicon oil versus mixtures of silicon oils with different viscosities supported this mechanism. Foam stability was much higher with the silicon oil mixtures, and similar dimpling also was observed.
Suja believes that this foam stability-driven evaporation is different than what is commonly observed in water-based lubricants. He says, “Water-based foam stability is directly related to the emulsifiers or surfactants, which tend to increase bubble stability by retarding film thinning. The evaporation mechanism we propose for oil-based lubricants that involves dimpling is much different.”
Suja and Fuller are now pursuing two strategies to find ways to reduce or eliminate foaming. The first is to formalize their understanding of bubble formation, evaporation and foaming with mathematical models that will allow them to simulate how pure or blended oils are likely to perform in real life. This would speed the research and discovery process for new lubricants that are less prone to foaming. The second is to look for antifoaming additives or other ways to counteract the evaporation driven foam stabilization.
Addition information can be found by contacting Dr. Fuller at
ggf@stanford.edu or Dr. Kar at
Abhishek.Kar@shell.com.
REFERENCES
1.
Canter, N. (2015), “FOAM: Dealing with a persistent problem,” TLT,
71 (12), pp. 24-38.
2.
Suja, V., Kar, A., Cates, W., Remmert, S., Savage, P. and Fuller, G. (2018), “Evaporation-induced foam stabilization in lubricating oils,”
Proceedings of the National Academy of Sciences,
115 (31), pp. 7919-7924.