Low-sulfur base oils have gained prominence during the past 25 years, but few suitable replacements have been found for sulfur-based additives for lubricants and metalworking fluids.
Today’s automotive fuels have almost no sulfur, so lubricant additives must make up for the beneficial properties that sulfur in fuels once provided.
Smarter sulfur' compounds can alleviate the negative effects of sulfur in lubricants, and non-sulfur compounds used alone or together with sulfur compounds can lower the overall sulfur content.
For many years sulfur compounds have been the go-to additives for a variety of functions in lubricants and metalworking fluids. However, regulatory limits on sulfur emissions and sulfur’s unwanted side effects have some people wondering whether sulfur-based additives should go away altogether.
New fluid formulations must keep pace with evolving demands for functionality. New engines are smaller and run hotter, and new fuel formulations—including biofuels—place new compatibility and durability demands on lubricants (
1). New materials and coatings require compatible, effective fluid additives for metalworking fluids during manufacturing and for lubricant additives after the vehicles or industrial equipment are in operation.
Sulfur compounds are very effective in providing this functionality. In older automotive fuel formulations, naturally occurring sulfur from sour crude oils once provided some degree of lubricity, even as these compounds contributed to air pollution and interfered with catalytic converters. However, current regulations place very low limits on the amount of sulfur allowed in fuels for passenger vehicles, heavy-duty vehicles and marine vessels, which puts a greater burden on lubricant additives to take up the slack.
Sulfur-containing automotive lubricant additives don’t pose the same pollution problems that high-sulfur automotive fuels once did. Engine oil can enter the combustion chamber where it is burned and released to the environment. However, the amount of combustion byproducts coming from engine oil is less than 1% of the amount coming from fuels, says STLE-member Mathias Woydt, who leads the Macrotribology and Wear Protection Group at the BAM Federal Institute for Materials Research and Testing in Berlin, Germany.
In metalworking fluids, sulfur compounds offer an even greater advantage: they are less toxic and more effective than the chlorinated compounds and barium detergent compounds they replaced.
Formulators have developed, and are continuing to work on, ash-free organosulfur compounds, additives that can be used in smaller amounts, and sulfur-based additives that can be coupled with non-sulfur compounds to reduce sulfur use still further. So rather than going away, sulfur-based additives may just be getting “smarter.”
Fuels and base oils
Combustion of high-sulfur petroleum fuels and coal releases sulfur oxide (SO
x) gases into the atmosphere. Electricity generation, metal smelting and other industrial processes are the largest sources of these gases. Vehicle exhaust was once a smaller but still significant source, but regulatory limits on the sulfur content of automotive fuels have greatly reduced on-road vehicle emissions as a source of SO
x (
2).
These regulatory limits were driven by the role of atmospheric sulfur compounds in producing acid rain, human respiratory problems and fine particulate matter pollution. Inorganic sulfur compounds also poison catalytic converters, causing them to lose efficiency and release more nitrogen oxide compounds into the air.
Government regulatory agencies, mostly in North America and Europe, continue to tighten limits on the sulfur content of fuels for automobiles, aircraft, marine vessels, off-road vehicles, power-generation utilities and home heating (
3). Today’s low-sulfur fuels may have less inherent lubricity because the refining process removes sulfur compounds along with other polar compounds that aid lubrication. Refining also removes naturally occurring antioxidants, so if these fuels are in contact with oxygen they can degrade, even when they are in storage (4).
Woydt notes that sulfur compounds in fuels weren’t all bad. “For engine oils, ash-forming [sulfur] compounds are bad for emissions, but they have good proven functionalities,” he says. Organic and inorganic sulfur compounds provide extreme pressure (EP) and antiwear (AW) protection, he continues, “so if you get rid of these for environmental reasons, you have to compensate on the materials side, with coatings, alternative base oils and additives, and so forth.”
The BAM Federal Institute for Materials Research and Testing is sponsored by the German Federal Ministry of Economics and Energy, Woydt explains. His group conducts applied research, mainly on automotive lubricants and industrial gear oils. “We have to show alternatives,” he says, before government agencies can implement a ban or lower limits on any given material or compound. He adds that these alternatives might not be on the market yet, but they must be “technically feasible, affordable and available, irrespective of their market share and penetration.”
A complicated balancing act
Coming up with viable alternatives to problematic sulfur compounds requires a series of functional and economic trade-offs. Ash-free formulations like polyglycol-based engine oils have only organic sulfur compounds, no inorganic ones, Woydt explains, but any loss in lubricity and wear protection requires that vehicle manufacturers invest in developing and implementing new materials and coatings on cylinder liners, piston rings, cams, tappets and other parts.
Ultra-low sulfur diesel fuels don’t affect the oxidative stability of engine oil, but biodiesel, which contains fatty acid methyl esters, can make engine oils more prone to oxidation. Biofuels, including biodiesel and bioethanol, also can promote corrosion, especially for parts made of copper or lead. On the other hand, acid compounds produced by the reaction of sulfur oxides with water and other compounds also can corrode engine parts.
Non-sulfur alternatives for use as lubricant additives are available, says STLE-member Vincent Gatto, research director for Vanderbilt Chemicals, but they are not as effective as the sulfur compounds. What you need is better sulfur chemistry and more efficient treat rates, he says. “The cost of making sulfur compounds, compared to other things, is very low,” he says. Alternatives to sulfur compounds are more expensive, and there is not yet a strong driver to take on the extra costs necessary to alleviate the harmful effects of sulfur compounds.
Will this change? “There are so many moving parts in formulating fluids, it all depends on where specifications head,” Gatto says. “There’s a strong driver to move to ashless materials, but it’s a balancing act,” he says, referring to the interplay between lubricant functionality, costs, new materials and technologies being used in engines and other mechanical systems, plus the constant push toward greater fuel economy. He foresees that certain sulfur chemistries will continue to be used because of the vital roles they play in the industry.
Vanderbilt makes a wide range of sulfurized additives, including ashless compounds and organometallics for use in industrial fluids, engine oils and greases, Gatto says. Ashless compounds are becoming increasingly important as ash products can interfere with sensors in diesel engines and poison catalytic converters.
A few starting compounds can be used to make a wide range of metal-containing and ashless additives. For example, dithiocarbamate (DTC) derivatives containing molybdenum, antimony or zinc are used in grease and engine oils, Gatto says (
see Figure 1). Molybdenum DTC additives are very effective friction modifiers, and they can improve fuel economy for commercial vehicles and passenger cars. “It’s hard to find alternatives for these materials because they are economical and effective, at least in the short term,” he says. Dimercaptothiadiazole (DMTD) derivatives, which his company also makes, are used mostly in grease applications.
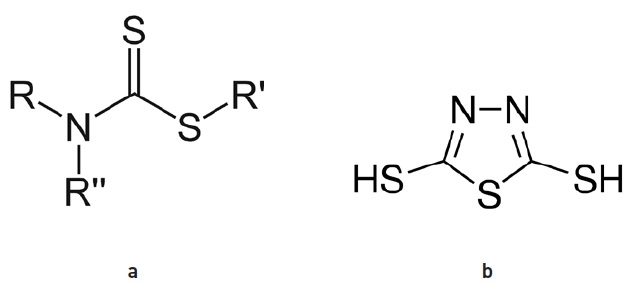 Figure 1. Dithiocarbamate (DTC) derivatives (a) containing molybdenum, antimony or zinc are used in grease and engine oils. Dimercaptothiadiazole (DMTD) derivatives (b) are used mostly in grease applications. (Figure 1a courtesy of Smokefoot, CC BY-SA 4.0; click here. Figure 1b courtesy of Sigma-Aldrich, Millipore Sigma.)
Figure 1. Dithiocarbamate (DTC) derivatives (a) containing molybdenum, antimony or zinc are used in grease and engine oils. Dimercaptothiadiazole (DMTD) derivatives (b) are used mostly in grease applications. (Figure 1a courtesy of Smokefoot, CC BY-SA 4.0; click here. Figure 1b courtesy of Sigma-Aldrich, Millipore Sigma.)
Corrosion is a major issue, Gatto says. The most problematic additives in this respect are sulfurized olefins like sulfurized isobutylene. These additives, which have high levels of chemically active sulfur, are being replaced, he says, especially in engine oils (
see Figure 2).
 Figure 2. Sulfurized isobutylene additives have high levels of chemically active sulfur. (Figure courtesy of U.S. Patent 6685824B2.)
Figure 2. Sulfurized isobutylene additives have high levels of chemically active sulfur. (Figure courtesy of U.S. Patent 6685824B2.)
Fluids for hydraulic equipment and gears still use these additives, but you have to use them properly. Gatto explains that proper usage involves optimizing the tradeoff between using high additive levels to enhance EP performance and limiting additive levels to reduce corrosion. If you get this balance wrong, you can solve one problem but cause another, he says.
This is why DMTD derivatives are replacing sulfurized olefins in some applications, Gatto continues. In particular, ashless dithiocarbamates are replacing sulfurized olefins in engine oils. Sulfurized olefins with a lower sulfur content are another option, he adds.
Small amounts of additives like tolutriazole corrosion inhibitors can keep sulfur-containing compounds from corroding yellow metals like copper, Gatto says (
see Figure 3). Although these additives do not react with the metal, they form a protective layer on the surfaces of metal parts that keeps the metal surfaces from coming into direct contact with the lubricant and its additives. He notes that fatty acids and other long-chain organic rust inhibitors, which protect ferrous metals by forming a barrier layer on the metal surface, are not effective in preventing sulfur corrosion on yellow metals.
 Figure 3. Tolutriazole corrosion inhibitors can keep sulfur-containing compounds from corroding yellow metals like copper. (Figure courtesy of Wikimedia Commons.)
Figure 3. Tolutriazole corrosion inhibitors can keep sulfur-containing compounds from corroding yellow metals like copper. (Figure courtesy of Wikimedia Commons.)
Sulfur scavengers are another, less common, option for corrosion prevention, Gatto says. These compounds react with active sulfur, converting it to less-aggressive chemical compounds that still retain their functionality. However, because these scavengers alter the chemistry of the lubricant, they can have a negative impact on performance. Scavengers also have to be used in greater amounts than do conventional corrosion inhibitors, and this increases costs.
Types of sulfur additives
The sulfur menagerie includes a wide variety of compounds that perform different functions and offer different strengths and limitations.
Elemental sulfur dissolved in mineral oil sometimes is used as an EP additive and is the most reactive sulfur-based additive. (In fact, it’s referred to as “active sulfur.”) Active sulfur reacts at a lower temperature than “inactive sulfur” compounds like sulfurized fatty acids, but it can corrode yellow metals and alloys.
Zinc dialkyldithiophosphate compounds (ZDDPs) are by far the most effective multifunctional antiwear agents. They form protective tribofilms that reduce wear, retard corrosion of the metal surface and trap free radicals and peroxides that cause lubricant oils to oxidize. Recent hardware developments make it possible to use less ZDDP, but these compounds still offer a unique combination of properties for which no true replacement has been found. The choice of the alcohols used in the preparation of the ZDDP determines the relative effectiveness of the ZDDP as an antiwear agent and its ability to withstand the effects of heat and water (
see Figure 4) (
1).
 Figure 4. Zinc dialkyldithiophosphate compounds (ZDDPs) are by far the most effective multifunctional antiwear agents. (Figure courtesy of Wikipedia; click here.)
Figure 4. Zinc dialkyldithiophosphate compounds (ZDDPs) are by far the most effective multifunctional antiwear agents. (Figure courtesy of Wikipedia; click here.)
ZDDPs aren’t perfect; they can interfere with the performance of other antioxidant additives, and they can form soluble and insoluble degradation products (
5). ZDDP additives also can interfere with the performance of catalytic converters, and this limits the amount that can be used in automotive lubricants.
Regulatory limits on sulfated ash, phosphorus and sulfur compounds, enacted to reduce harmful emissions, is another factor limiting ZDDP use in automotive applications (
6). It’s worth noting that lubricant additives like ZDDPs and molybdenum disulfide (MoS
2) don’t pose a significant risk to humans, but they do not biodegrade readily and may persist in the environment (
7).
Molybdenum dithiocarbamate, another tribofilm-forming compound, is widely used in engine oils where it reduces friction in the boundary lubrication regime. This compound, which is often used in greases as well, forms a low melting point eutectic that is softer than the metal itself.
Molybdenum dithiocarbamates, molybdenum dithiophosphates and other more complex molybdenum compounds are extensively used for friction modification. These compounds react on the metal surface to yield MoS
2, which has a structure that allows sliding and shearing to take place (
1). Some organic molybdenum compounds may degrade diamond-like coatings (DLCs)—amorphous hydrogenated carbon films—that reduce friction and protect metal parts in some newer engines, Woydt says.
Molybdenum disulfide (MoS2), an old standby solid lubricant, provides good lubrication and EP load-carrying capacity. Woydt notes that products based on a dispersion of MoS
2 and graphite have been on the market for many decades. During World War II, he adds, the British Air Force used MoS
2-based additives to help their airplanes continue to fly for a while after they had been hit and were losing engine oil.
Woydt notes that MoS
2 discharged through the engine oil into the exhaust produces ash that can clog particulate filters. When MoS
2 is oxidized to MoO
3, it can diffuse into zirconia-based oxygens sensors and interfere with the signal (
see Figure 5).
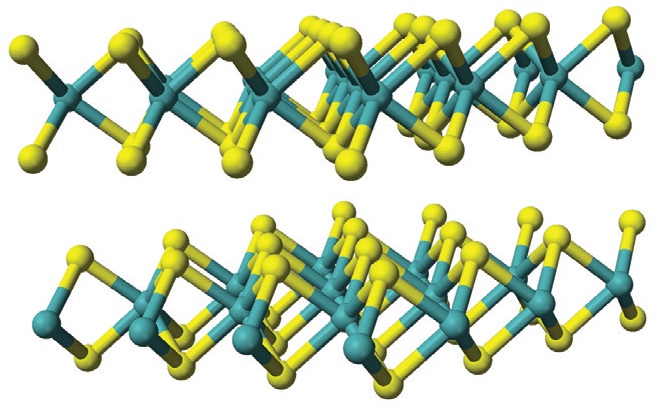 Figure 5. Molybdenum disulfide’s layered structure provides good lubrication and EP load-carrying capacity, but this additive may harm coatings in newer engines. (Figure courtesy of Wikimedia Commons.)
Tungsten disulfide
Figure 5. Molybdenum disulfide’s layered structure provides good lubrication and EP load-carrying capacity, but this additive may harm coatings in newer engines. (Figure courtesy of Wikimedia Commons.)
Tungsten disulfide (WS
2) is chemically similar to its molybdenum counterpart. Both sulfide compounds have similar flat layered crystal structures, and they also can form inorganic fullerenes (IFs): soccer-ball-shaped cage structures that resemble C
60 “buckyballs” (
see Figure 6). At least one company, NanoLub, offers commercial products, including bearing oils, metalworking fluids and other fluids that incorporate multilayered IF-WS
2 nanospheres that they claim reduce friction and mechanical wear.
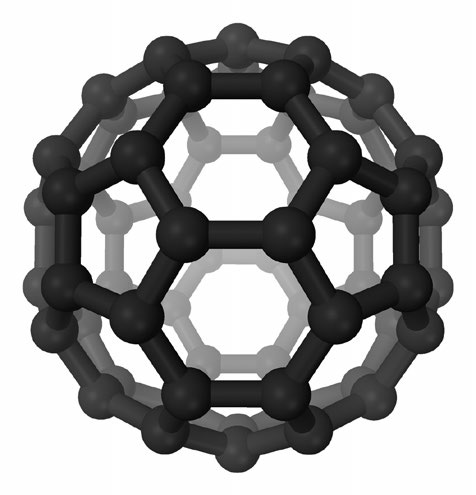 Figure 6. Buckminsterfullerene, also known as “buckyballs,” is a phase of pure carbon that takes the form of a 60-cornered cage. Some inorganic compounds, like WS2, form cage-like structures sometimes referred to as “inorganic fullerenes.” (Figure courtesy of Wikimedia Commons.)
Sulfonate detergents
Figure 6. Buckminsterfullerene, also known as “buckyballs,” is a phase of pure carbon that takes the form of a 60-cornered cage. Some inorganic compounds, like WS2, form cage-like structures sometimes referred to as “inorganic fullerenes.” (Figure courtesy of Wikimedia Commons.)
Sulfonate detergents reduce deposits and protect against corrosion and rust (
see Figure 7). Overbased detergents can neutralize the mineral acids formed by fuel combustion and the organic acids formed by oil oxidation. Calcium sulfonates are generally not hazardous, but they can irritate the skin (
7).
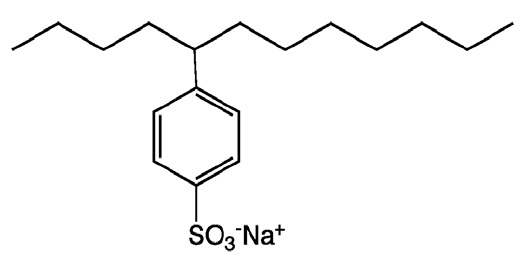 Figure 7. Sulfonate detergents reduce deposits, and they protect against corrosion and rust. (Figure courtesy of Wikipedia; click here.)
Figure 7. Sulfonate detergents reduce deposits, and they protect against corrosion and rust. (Figure courtesy of Wikipedia; click here.)
Overbased calcium and sodium sulfonates, which are used as EP additives, contain colloidal particles of carbonate salts dispersed through the sulfonate phase. The colloidal particles react with iron to form a barrier film between the metal surfaces (
8).
Other organosulfur additives include sulfurized fats and esters (friction modifiers), sulfurized hydrocarbons and polysulfide compounds. Sulfurized additives used with neat-oil and water-dilutable metalworking fluids are especially useful for processing aluminum alloys (
8).
In fact, sulfur-containing additives are still considered essential in metalworking fluids. They have replaced the more harmful chlorinated paraffins, which can release bio-accumulating carcinogens, including furans and dioxins, when used oils containing these compounds are incinerated in power plants or cement kilns.
Sulfur compounds, which react with metal surfaces to form protective films, perform better than chlorine compounds at high interfacial temperatures. Sulfate ions can affect the ability of a metalworking fluid to prevent rust, but this effect is not as severe as for chloride ions (
9).
Natural sodium petroleum sulfonate was a widely used metalworking fluid emulsifier until 2003 when a major supplier closed its plant, driving a switch to synthetic sulfonates and other compounds (
9). Sulfur-free biodegradable chlorinated esters and fatty acids, polyol ester compounds and compounds based on phosphorus or nitrogen are other alternatives to chlorinated paraffins (
10).
Ash or no ash
It’s worth noting the distinctions between organosulfur compounds, organometallic sulfur compounds and inorganic sulfur compounds. Inorganics and organometallics produce ash, which is the source of the problem for tailpipe emissions, catalytic converter poisoning and clogged filters.
Pure organic additive packages contain no salts or organometallics (but may include organosulfur compounds). Organosulfur additives that do not contain metallic elements are ash-free, and they can be an environmentally benign alternative, Woydt says. These compounds can be used in the newer engines, and because lubricant byproducts are emitted to the atmosphere in such small amounts, they cause minimal pollution, he adds.
Organometallics offer better protection against scuffing at high temperatures because they form a solid tribofilm on the rubbing surfaces, but even here, there are a variety of options, including nontoxic bismuth compounds, Woydt says. Organometallics do produce ash, but this seems a small price to pay for the 1%-1.5% increase in fuel economy that molybdenum compounds, for example, can provide, he adds.
Automotive equipment manufacturers would probably be receptive to using ash-free lubricants, he continues, but putting the whole lubricant package together to form a successful commercial product can be a complicated proposition. Equipment manufacturers have to use materials that are compatible with the new lubricants and additives, which requires investment in testing and adaptation, as well as user training. “The question of miscibility and backward compatibility also is on the table,” he says. He adds that new players coming into the business may be more willing to make these investments, citing DLCs as one example of a spreading technology.
Moving beyond sulfur
Most commercial replacements for sulfur additives are boron compounds and sulfur-free molybdenum compounds, Gatto says. These can be used in combination with sulfur-containing additives to reduce the total amount of sulfur in a formulation, which helps reduce corrosion, he adds. Although boron-based additives have been around for a long time, “developing new chemistry takes a very long time in our industry,” Gatto says. New formulations must not only be effective, they must comply with regulations, not pose too many environmental issues and be amenable to production at sufficient volumes to make them worthwhile.
Organic compounds, with or without sulfur, are an ash-free alternative to inorganic and organometallic compounds. One common example, salicylates, are sulfur-free detergents that protect against wear at least as well as ZDDP/borate ester additives under the right conditions (
11), and they function better as friction modifiers than glycerol mono-oleates and oleylamides (
1).
Boron compounds are another well-established class of lubricant additives. These compounds are readily available, have a low degree of toxicity and do not produce offensive odors. They provide efficient antiwear protection, good film strength and self-lubrication properties (
12).
Inorganic borate particles in mineral oil stand up well under high temperatures and heavy loads at the same time, unlike sulfur-containing gear additives, which can tolerate one or the other, but not both. In addition, inorganic boron compounds serve as fire retardants (
12).
Boric acid and boron nitride both have layered crystal structures (
see Figure 8). However, boric acid is easily sheared and forms a self-lubricating solid, while boron nitride needs an oxided metal surface layer to be self-lubricating. Boric acid can decompose to boric oxide and water at high temperatures (
12). Boric acid was once commonly used in metalworking fluids because of its EP, AW and bacteriocidal properties. However, concerns over its health, safety and environmental effects caused it to be phased out of these applications (
13).
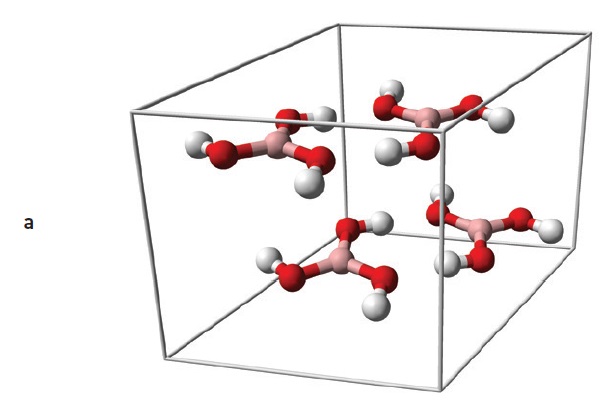
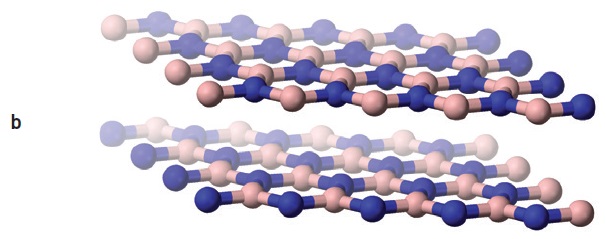
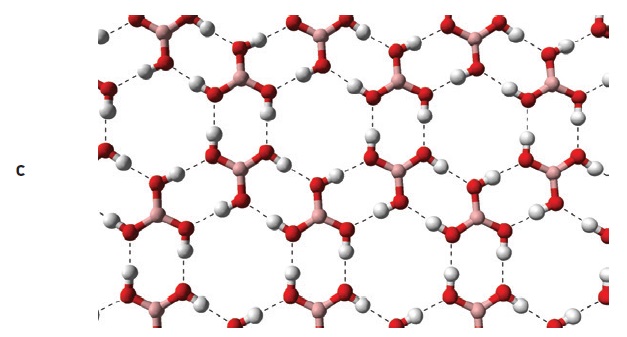
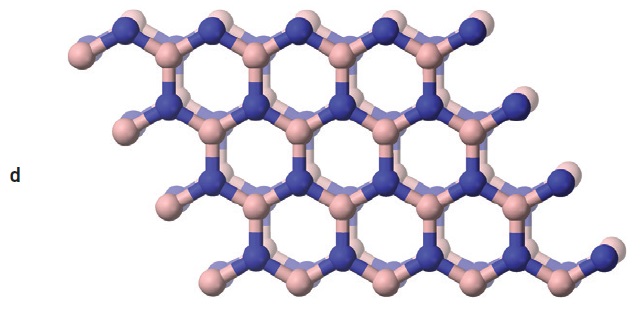 Figure 8. Boric acid (a) and boron nitride (b) have similar layered structures, but boric acid layers (c) are formed from hydrogen-bonded molecules, while boron nitride layers (d) are chemically bonded networks. (Figure courtesy of Wikimedia Commons.)
Figure 8. Boric acid (a) and boron nitride (b) have similar layered structures, but boric acid layers (c) are formed from hydrogen-bonded molecules, while boron nitride layers (d) are chemically bonded networks. (Figure courtesy of Wikimedia Commons.)
Some formulators of automotive and industrial lubricants are investigating additives containing boron-based nanoparticles (especially nanoparticulate boric acid) as an alternative to additives based on sulfur or phosphorus compounds. However, boric acid does not offer the antioxidant properties that ZDDP does, and it is incompatible with some engine oil additives (e.g., total base number buffers), which can lead to corrosion and sludge formation. Drivetrain components in advanced wind turbines might benefit from colloidal boron nitride, along with electrochemical boriding treatments, but the jury’s still out on this (
13).
Organoborates work synergistically with oil-soluble compounds to offer better antiwear performance than any of the individual components alone, and they also act as antioxidants. One type of organoborates, the borate esters, have been shown to form amorphous films on ferrous metals, composed of boron and ferrous and organic compounds. Steel surfaces coated with borate ester EP films, which do not shear easily, are harder than uncoated surfaces. When borate esters form condensation polymers with diols, the result is a non-sacrificial boron-containing solid film that does not react with the metal surface. Borate esters are prone to hydrolysis (which can form oil-insoluble, abrasive boric acid crystals). Amine compounds or hindered phenols can stabilize borate esters, and diol compounds can convert boric acid decomposition products into stable ring structures (
12).
Nanoparticles are a promising field of research for surface protection and friction reduction, but the gap between the lab and the marketplace can prove to be problematic. Numerous studies have shown that nanotechnology can improve the performance of oils and greases, but technical and legislative obstacles, along with difficulties in integrating nanoparticles into complete lubricant packages, hinders the development of viable commercial products (
13).
Early studies claimed that nanodiamonds (4-6-nm particle size) embed into sliding surfaces or provide rolling lubrication, but more recent tests show that they reduce friction by micropolishing the surfaces, which reduces running-in time. Nanodiamonds lose their effectiveness in aged oil, where wear rate and steady-state surface roughness are less important than oil contamination and other factors. Nanodiamonds continue to polish the surface even after the running-in phase, which may lead to excessive wear over time (
see Figure 9) (
13).
 Figure 9. Nanodiamonds may reduce friction by micropolishing metal surfaces during the run-in period. (Figure courtesy of D. Mukherjee, CC BY-SA 4.0; click here.)
Fullerenes
Figure 9. Nanodiamonds may reduce friction by micropolishing metal surfaces during the run-in period. (Figure courtesy of D. Mukherjee, CC BY-SA 4.0; click here.)
Fullerenes, soccer-ball shaped carbon cages, would seem to be a less abrasive source of rolling lubrication. However, claims of rolling lubrication for fullerenes (most commonly C
60) and inorganic fullerene-like (IF) materials (e.g., IF-WS
2) haven’t been proven conclusively. In addition, much remains to be documented about the health, safety and environmental effects of fullerenes. IF-WS
2 has poor oxidation stability, and it corrodes copper (
13).
Polytetrafluoroethylene (PTFE) would seem to be an ideal lubricant (
see Figure 10). This polymer has been used as a dry-film lubricant and friction modifier, and PTFE-fortified oils and greases do exhibit higher welding loads, higher load wear indexes and reduced stick-slip. Several aftermarket engine treatment products incorporate PTFE nanodispersions, but these dispersions are not stable in engine oil, and they can clog oil filters and present problems with recycling. DuPont, the company that manufactures Teflon brand PTFE, actually discourages using PTFE in engine oils (
13). Claims that PTFE-enhanced commercial lubricants improve engine performance and reduced engine wear have not been substantiated (
14).
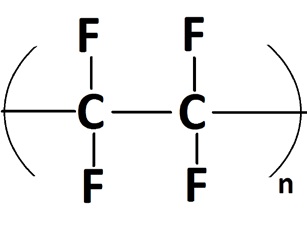 Figure 10. PTFE, a very effective nonstick surface coating, may not be appropriate for use as a lubricant additive. (Figure courtesy of Wikimedia Commons.)
Silicate compounds
Figure 10. PTFE, a very effective nonstick surface coating, may not be appropriate for use as a lubricant additive. (Figure courtesy of Wikimedia Commons.)
Silicate compounds might be one viable alternative to phosphorus and sulfur-based additives for metalworking fluids. Silicates do not serve as nutrients for microbes, and they work very well if steps are taken to prevent the formation of insoluble gels (
15).
Isosteric compounds are another promising alternative. This approach to reducing or eliminating sulfur from lubricant additives involves synthesizing chemical compounds that are structurally similar to common sulfur-containing additives, but replacing the some or all of the sulfur atoms with oxygen or nitrogen atoms.
Gao et al. (
16) synthesized a series of potential automotive lubricant additives using thiobenzothiazole or 2-benzothiazole-S-carboxylic acid esters as templates. They performed tribological testing on these compounds, which demonstrated similar or improved results compared with the sulfur template compounds.
Smarter sulfur
Government regulations mandate strict limits on the sulfur content of diesel fuels, and modern base oils have inherently low levels of sulfur. There appears to be less of a regulatory push for reducing or removing the sulfur from lubricant additives, Gatto says. The sulfur in additives provides a necessary function at a low cost, so for the time being there’s no strong impetus to change this. The industry is moving from Group I base stocks to the lower-sulfur (or sulfur-free) Group II and Group III base stocks, which have less inherent lubricity and corrosion protection properties, so that’s done with additives now, he says.
The key challenge is to use sulfur efficiently and effectively, he adds. In this respect, lubricant research on sulfur additives is following a course similar to previous initiatives for phosphorus-based additives: lower levels and fewer detrimental side effects, while preserving the effective performance characteristics. New additives can have a lower sulfur content, but they must be able to perform well in existing and upcoming applications, Gatto says. This is especially important in commercial applications, where equipment owners are not willing to reinvest in new equipment every few years.
REFERENCES
1.
Technical Committee of Petroleum Additive Manufacturers in Europe (ATC), Document 118, August 2016, Lubricant Additives: Uses and Benefits. Available
here.
2.
U.S. EPA Report on the Environment, Sulfur Dioxide Emissions, 2014. Available
here.
3.
Kishore Nadkarni, R. A. (June 2004), “The Challenge of Sulfur Analysis in the Fuels of the Future,”
ASTM Standardization News. Available
here.
4.
Gupta, S. (Dec. 7, 2016), “A low sulphur future,”
Wilhelmsen Insights. Available
here.
5.
Johnson, M. (2008), “Selecting the correct lubricant,” TLT,
64 (3), pp. 28-36.
6.
Canter, N. (2008), “Use of antioxidants in automotive lubricants,” TLT,
64 (9), pp 12-19.
7.
Madanhire, I. and Mbohwa, C. (2016), “Chapter 2: Lubricant Additive Impacts on Human Health and the Environment,”
Mitigating Environmental Impact of Petroleum Lubricants, Springer International Publishing Switzerland, pp. 17-34. DOI: 10.1007/978-3-319-31358-0_2. Available
here.
8.
Canter, N. (2007), “Trends in extreme pressure additives,” TLT,
63 (9), pp. 10-17.
9.
Byers, J. P., ed. (2006),
Metalworking Fluids, second edition, CRC Press.
10.
Van Rensselar, J. (2015), “The future of metalworking fluid additives,” TLT,
71 (3), pp. 37-45.
11.
Maurya, J. L., Jaiswal, V. and Rastogi, R. B. (2015), “Highly efficient sulfur and phosphorous-free antiwear additives for paraffin oil,”
Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology,
230 (2), pp. 222-237. Available
here.
12.
Choudhary, R. B. and Pande, P. P. (2002), “Lubrication Potential of Boron Compounds: An Overview,”
Lubrication Science,
14 (2), pp. 211-222.
13.
Zhmud, B. and Pasalskiy, B. (2013), “Nanomaterials in Lubricants: An Industrial Perspective on Current Research,”
Lubricants,
1, pp. 95-101. DOI:10.3390/lubricants1040095.
14.
U.S. Federal Trade Commission, (July 16, 1996), “Quaker State Ads for Slick 50 are False and Misleading, FTC Charges,” press release. FTC File No. 932 3050, Docket No. D-9280. Available
here.
15.
Kim, H. and Fachini, A. (2016), “Influence of inhibitor’s chemistry and structure on aluminum corrosion in MWF,” STLE Annual Meeting, Las Vegas, Nev., May 15-19, 2016, Metalworking Fluids.
16.
Gao, X., Liu, D., et al. (2018), “Isosteric design of friction-reduction and anti-wear lubricant additives with less sulfur content,”
Friction,
6 (2), pp. 164-182. Available
here.