A new analytical technique, X-ray ptychographic tomography, was used to resolve how nanoparticles undergo redox reactions in a lithium-ion battery.
Heterogeneous particles were identified that are undergoing redox reactions at different rates.
This technique should be useful in understanding what makes a good battery electrode and troubleshooting battery failures in the future.
The move toward using battery technology in applications such as automobiles has focused on ways to improve the performance of high-energy lithium types. As this column has noted, flammability and toxicity continue to be major safety issues when working with lithium-ion batteries.
In a previous TLT article, researchers reported the development of a new cathodic material based on lithium, iron and oxygen that can theoretically double the energy density of the battery compared to the material currently used, lithium cobalt oxide (
1). The researchers determined that the new cathodic material was formed irreversibly from a related lithium iron oxide.
One approach that may assist researchers in determining the potential concerns about using lithium-ion batteries is to find out what reactions occur within the battery on the nanoscale in three dimensions. Jordi Cabana, associate professor of chemistry at the University of Illinois at Chicago, says, “Two well-known analytical techniques are available to evaluate reactions at the nanoscale. X-ray imaging methods have proven to be effective in evaluating chemical reactions in bulk media but are limited due to their inability to resolve materials with diameters less than 30 nanometers. Electron-based imaging techniques exhibit atomic scale resolution, but they are limited in not being able to resolve materials that are thicker than 100 nanometers.”
The other problem is both techniques most often only provide an analysis of materials in two dimensions. To obtain an accurate assessment of how battery materials are engaging in redox reactions requires an analysis of the materials in three dimensions to differentiate surface and inner processes. Cabana says, “This factor is particularly important because battery electrodes are clearly heterogeneous mixtures, and the active materials change internally in a heterogeneous manner.”
A new approach is needed to accurately evaluate how particles moving through a lithium-ion battery are engaged in redox processes in three dimensions. Such a process has now been developed.
X-ray ptychographic tomography
Cabana and his research group, in collaboration with colleagues at the Lawrence Berkeley National Laboratory, devised an analytical technique known as X-ray ptychographic tomography to resolve nanoparticles undergoing redox reactions in a battery down at a resolution of approximately 11 nanometers. He says, “In X-ray ptychographic tomography, a coherent, nanoscale beam of X-rays is generated by the high-flux synchrotron accelerator at Lawrence Berkeley National Laboratory’s Advanced Light Source. The beam can be moved a few nanometers at a time, followed by sample rotation, to produce three-dimensional chemical maps of the nanoparticles involved in redox reactions.”
The researchers evaluated lithium iron phosphate as the cathode in this study. Nanoplates of lithium iron phosphate were prepared through reaction of phosphoric acid with lithium hydroxide monohydrate and ferric sulfate heptahydrate at 180 C for 10 hours. The nanoplates were then carbon coated with 20 weight percent sucrose followed by carbonizing at 650 C for three hours in an argon atmosphere.
Cabana says, “Lithium iron phosphate is a well-known cathodic material that has been used in batteries for some time. Once we prepared this material, our approach was to extract it from a lithium metal half-cell that had been 50% delithiated and use X-ray ptychographic tomography to examine the particles in a half-charged battery.”
The researchers evaluated 83 individual nanoparticles harvested from this electrode in a recent paper (
2). To minimize error and enhance resolution, the particles were organized into three categories: greater than 70% alpha lithium iron phosphate (rich in ferrous ions), greater than 70% beta lithium iron phosphate (rich in ferric ions) and a 30%-70% mixture of both compounds.
Cabana says, “In the discharge reaction, ferrous ions are converted to ferric ions. But the process does not progress in a uniform manner. Rather, we detected heterogeneous particles (as shown in a three-dimensional chemical map in Figure 2) that contain percentages of all chemical categories. This process can be considered analogous to ice melting to form water. Ice does not completely convert to water at the melting point all at once but gradually with both solid and liquid phases present.”
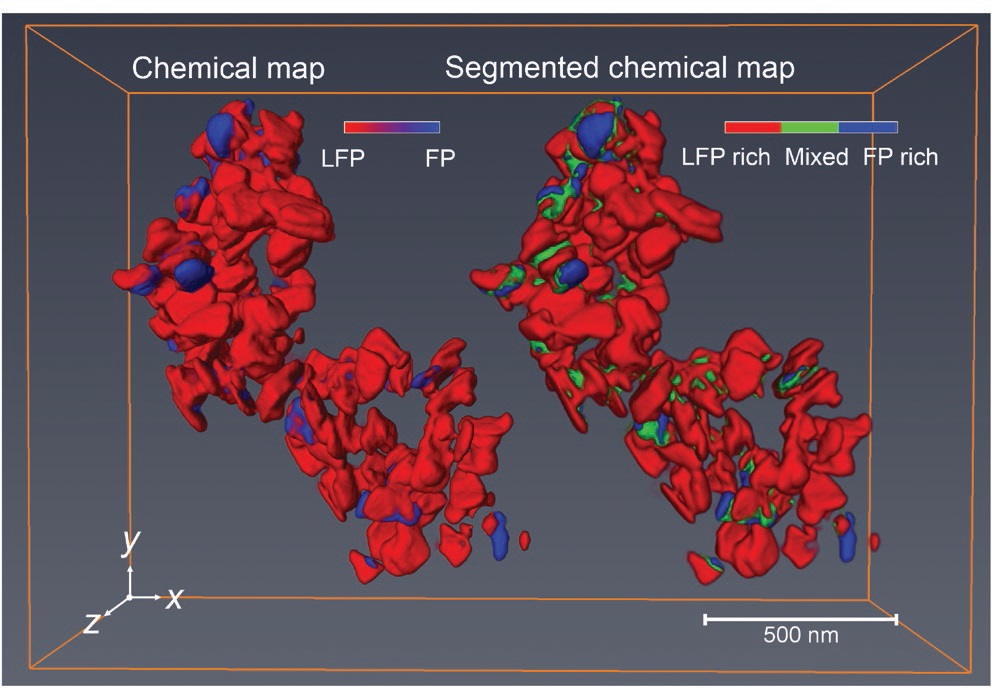 Figure 2. A three-dimensional chemical map displays three categories of particles identified in a lithium-ion battery. The primary one shown is LFP, which is rich in ferrous ions. (Figure courtesy of the University of Illinois at Chicago.)
Figure 2. A three-dimensional chemical map displays three categories of particles identified in a lithium-ion battery. The primary one shown is LFP, which is rich in ferrous ions. (Figure courtesy of the University of Illinois at Chicago.)
One other point made by Cabana has to do with heterogeneity. He says, “The more heterogeneity seen in battery materials, the greater chance that battery instability can be induced through inadvertent overcharging/discharging.”
Cabana believes that the data shows the discharge reaction is not taking place at the same reaction rate in the nanoparticles. He says, “Think of runners in a marathon when considering why particles are not reacting at the same rate. Some runners move faster than others. Factors affecting the rate of reaction include the physical orientation of the battery. One consideration is that lithium ions and electrons involved in the redox reaction are not occupying the same space and not moving at the same rate. Their ability to diffuse has a direct impact on the rate of reaction.”
The researchers have developed a tool to study battery reactions, which should provide a technique for helping to understand what makes a good battery electrode and troubleshoot battery failures in the future. Cabana says, “We intend to examine the performance of the currently used layered oxide cathodic materials by combining lithium with various metals such as nickel, manganese and cobalt.
Additional information can be found in the recently published paper (
2) and by contacting Cabana at
jcabana@uic.edu.
REFERENCES
1.
Canter, N. (2018), “Higher-performing lithium-ion batteries,” TLT,
74 (3), pp. 14-15.
2.
Yu, Y., Farmand, M., Kim, C., Liu, Y., Grey, C., Strobridge, F., Tyliszczaki, T., Celestre, R., Denes, P., Joseph, J., Krishnan, H., Maia, F., Kilcoyne, A., Marchesini, S., Leite, T., Warwick, T., Padmore, H., Cabana, J. and Shapiro, D. (2018), “Three-dimensional localization of nanoscale battery reactions using soft X-ray tomography,”
Nature Communications,
9, Article Number: 921.
.