Enhancing the rate of oxygen reduction in fuel cells
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2018
A new catalyst coating on the cathode reduced strontium migration.
 © nissannews.com
© nissannews.com
KEY CONCEPTS
•
Strontium migration to the surface leads to the degradation of the LSCF cathode of a solid oxide fuel cell.
•
A multi-phase catalyst coating applied to the LSCF cathode reduced strontium migration and improved the rate of oxygen reduction in the fuel cell.
•
Under operating conditions of 600 C and a constant cell voltage of 0.7 V, degradation was observed with the uncoated LSCF cathode while no performance change was observed with the coated LSCF cathode after 250 hours of operation.
to be the subject of research as an alternative propulsion source to the internal combustion engine. The appeal of using fuel cells is that a higher energy efficiency can be achieved.
Efforts to commercialize fuel cells are continuing, but there are challenges to overcome. In a previous TLT article, modifications to a fuel cell containing a proton exchange membrane based on copolymers of perfluorosulfonic acid and perfluoroethylene were described (
1). The researchers dispersed tungsten carbide nanoparticles in the copolymer matrix, which served to improve fuel cell durability and reduce cost. The latter was due to the tungsten carbide replacing platinum.
A solid oxide fuel cell uses a solid oxide electrolyte and ceramic or ceramic-metal composite electrodes. The key reaction taking place at the cathode is the reduction of oxygen gas from the air to the oxygen anion, O
2-. Meilin Liu, Regents’ Professor in Georgia Tech’s School of Material Science and Engineering in Atlanta, says, “This process, known as the oxygen reduction reaction, involves several distinct steps. Initially molecular oxygen adsorbs on the surface of the cathode followed by dissociation of oxygen molecules to monoatomic oxygen species through the use of transition metal oxide catalysts. Surface diffusion follows, moving the oxygen species to vacant sites in the catalyst where partial reduction takes place. Then the oxygen species jumps through the vacant site to the other end of the fuel cell to react with hydrogen and produce water.”
According to Liu, the state of the art cathode under evaluation is an oxide based on lanthanide, strontium, cobalt and iron that is known as LSCF. He says, “The LSCF cathode is very effective in the temperature range (600-800 C), but performance issues are present due to strontium segregation.”
When the LSCF cathode of a solid oxide fuel cell is exposed to air containing carbon dioxide and moisture, strontium tends to migrate to the surface, leading to the formation of insulating phases that degrade the catalytic activity of the cathode. Liu says, “A noticeable reduction in solid oxide fuel cell performance is seen after thousands of hours of operation due to this degradation.”
To eliminate this problem and enable solid oxide fuel cells to operate at least over a five-year period, a new approach is needed to minimize cathode degradation. Such an approach has been developed.
Multi-phase catalyst coating
Liu, Dr. Yu Chen, postdoctoral research associate, and their colleagues modified LSCF through the preparation of a multi-phase catalyst coating that significantly increased the performance and durability of the cathode, leading to improved solid oxide fuel cell performance. Liu says, “We found that by applying a specific coating containing segregated nanoparticles consisting of oxides of barium and cobalt (BaCoO
x) and oxides of praseodymium and cobalt and a thin film containing oxides of praseodymium, barium, calcium and cobalt (PBCC), we significantly improved the rate of the oxygen reduction reaction and the durability of the cathode.”
The researchers designated this heterogeneous catalyst as a multi-phase catalyst coating. Liu says, “The coating exhibited a thickness of 10-20 nanometers, and the oxide nanoparticles were about 5-10 nanometers in diameter.”
A schematic of the multi-phase catalyst coating is shown in Figure 1. Microanalyses revealed the composition of the coating and showed that two types of nanoparticles were present within the coating.
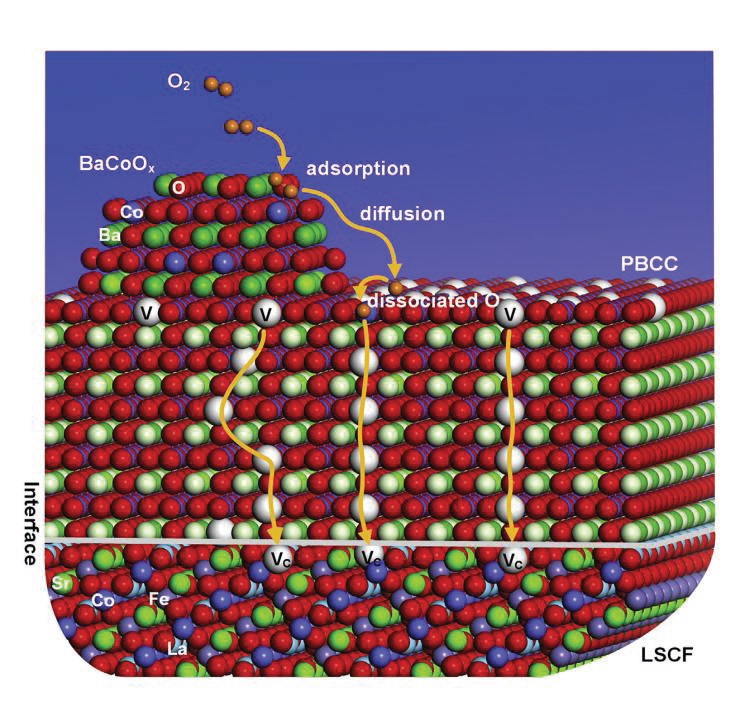 Figure 1. A multi-phase catalyst consisting of nanoparticles of oxides of barium and cobalt (BaCoOx) and a thin film containing oxides of praseodymium, barium, calcium and cobalt (PMCC) facilitated the movement of oxygen through vacant (V) sites into the LSCF cathode of a solid oxide fuel cell. (Figure courtesy of Georgia Tech.)
Figure 1. A multi-phase catalyst consisting of nanoparticles of oxides of barium and cobalt (BaCoOx) and a thin film containing oxides of praseodymium, barium, calcium and cobalt (PMCC) facilitated the movement of oxygen through vacant (V) sites into the LSCF cathode of a solid oxide fuel cell. (Figure courtesy of Georgia Tech.)
Liu says, “The multi-phase catalyst coating enhanced the oxygen reduction reaction and acted to prevent strontium segregation even in the presence of carbon dioxide and water. The thickness of the coating is critical to ensure performance. Too thin of a coating could have limited durability, while too thick of a coating might result in reduced performance due to increased resistance to ion transport.”
Standard techniques were used to evaluate the electrochemical performance of the multi-phase catalyst coated LSCF cathode. Using a model cell, the researchers showed that the coated LSCF displayed a one order of magnitude reduction in polarization resistance compared to LSCF. Liu says, “Impedance spectroscopy showed that the cathode with the multi-phase catalyst coating showed greater tolerance to contaminants and smaller resistance to oxygen reduction.”
Durability testing was conducted by evaluating the coated and uncoated LSCF cathode at 600 C under a constant cell voltage of 0.7 V. Performance degradation was observed with the uncoated LSCF cathode after 250 hours of operation. But the cathode with the multi-phase catalyst coating showed no change in performance.
The researchers intend to optimize the performance of the multi-phase catalyst coating in the future and also develop a similar strategy to upgrade anode performance. Liu says, “The LSCF cathode is not yet suitable for use in automobile fuel cells because it is not effective at a lower operating temperature of 500 C. We believe that, as new materials are developed, the fuel cell has potential for automotive applications because it exhibits a greater energy density than batteries. Ultimately the best solution for plug-in electric vehicles might be a combination of the fuel cell and the battery.”
Additional information can be found in a recent article (
2) or by contacting Liu at
meilin.liu@mse.gatech.edu.
REFERENCES
1.
Canter, N. (2017), “More durable and cost-effective fuel cell,” TLT,
73 (12), pp. 12-13.
2.
Chen, Y., Choi, Y., Yoo, S., Ding, Y., Yan, R., Pei, K., Qu, C., Zhang, L., Chang, I., Zhao, B., Zhang, Y., Chen, H., Chen, Y., Yang, C., deGlee, B., Murphy, R., Liu, J. and Liu, M. (2018), “A Highly Efficient Multi-phase Catalyst Dramatically Enhances The Rate of Oxygen Reduction,”
Joule,
https://doi.org/10.1016/j.joule.2018.02.008.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.