PAGs: Reducing ester vulnerability to hydrolysis
Dr. Neil Canter, Contributing Editor | TLT Tech Beat October 2017
An ethylene oxide/propylene oxide triblock copolymer traps water like a sponge.
KEY CONCEPTS
•
Use of a specific PAG architecture, triblock copolymer, dramatically improves the hydrolytic stability of vegetable oils and synthetic esters.
•
These PAGs display a significant viscosity increase in the presence of water.
•
The effectiveness of the triblock copolymers might be due to their ability to act as polymeric sponges to absorb and trap water in lubricant compositions.
ESTERS HAVE FOUND WIDESPREAD USE in lubricants, and there is growing interest in using them for environmentally acceptable lubricants where their biodegradability meets existing regulatory requirements. While exhibiting good lubricity, an important class of ester-based lubricants, vegetable oils unfortunately are vulnerable to oxidation due to the presence of high levels of monounsaturates and polyunsaturates in the triglyceride structure.
In a previous TLT article, researchers blended a specific vegetable oil known as high oleic canola oil with a polyalkylene glycol (PAG) (
1). Evaluation of the oxidation and sludge generation characteristics of this blend showed it to be superior to the canola oil. The polar PAG showed improved deposit control by helping to solubilize oxidation byproducts of the ester, thereby keeping the fluid cleaner and extending fluid life.
One other concern with using esters is hydrolytic stability. This vulnerability has become more apparent in the U.S. with the implementation of the Vessel General Permit (VGP) regulation by the EPA in December 2013. VGP requires that environmentally acceptable lubricants be used in all applications where there is an interface between the lubricant and the sea. Further details on the regulation can be found in a recent article (
2).
But contact between the lubricant and the sea can lead to premature failure due to hydrolysis. STLE-member Dr. Martin Greaves, research leader at The Dow Chemical Co. in Horgen, Switzerland, says, “Vegetable oils and synthetic esters are used in VGP applications such as hydraulic fluids, gear lubricants, greases and stern tube greases. We evaluated the hydrolytic stability of two vegetable oils (canola oil and sunflower oil) and three synthetic esters (estolide, saturated trimethylolpropane [TMP] ester and TMP trioleate) using a modified ASTM D2619.”
In this procedure, the esters are heated for 48 hours at 93 C in the presence of 10% water and a copper coupon. An amine phosphate catalyst was added at 0.25% to artificially accelerate the hydrolysis.
Greaves says, “Without the catalyst, the esters are fairly stable and slow to hydrolyze. In practice, esters can oxidize to form acidic components (or there is free acid remaining from the ester manufacturing process) and it is this acid that we believe accelerates ester hydrolysis in the presence of water.”
The researchers measured hydrolysis by evaluating the increase in total acid number (TAN) for each ester. Results for the four esters show TAN increases ranging from 2.5-8.0 milligrams of potassium hydroxide per gram of ester.
If an approach can be found to reduce the increase in TAN, then esters can more readily be used in water-sensitive applications such as marine lubricants. Such a strategy has now been developed.
PAG TRIBLOCK COPOLYMER
Greaves and his colleagues determined that addition of a specific PAG architecture up to a concentration of 10% in the vegetable oils and synthetic esters dramatically improves their hydrolytic stability. He says, “We decided to evaluate the use of PAGs because we know that PAGs are hygroscopic, but the water absorbed is latent water up to the polymer’s saturation point. In other words, it is not ‘free’ water but bound by the polymer structure through what we believe are simple hydrogen bonds. Our hypothesis was that carefully designed PAGs may be able to trap ‘free’ water within the polymer structure and render it inert or latent.”
The researchers evaluated four types of PAG chemistries, including a homopolymer (polypropylene glycol monobutyl ether) and three types of copolymers (random, block and reverse block). Although homopolymers and random copolymers are widely used in modern lubricants, blocks and reverse blocks are much less common and not so well researched. They found that an ethylene oxide/propylene oxide triblock copolymer improved the hydrolytic stability of the vegetable oils and synthetic esters by reducing the increase in TAN as shown from the ASTM D2619 test method.
For example, Figure 1 shows the improvement in hydrolytic stability for canola oil when treated with a triblock copolymer that has an ethylene oxide (EO), propylene oxide (PO) ratio of 30:70. A significant reduction in TAN increase is detected compared to a triblock copolymer with an EO:PO ratio of 10:90, two PAG random copolymers and polypropylene glycol monobutyl ether.
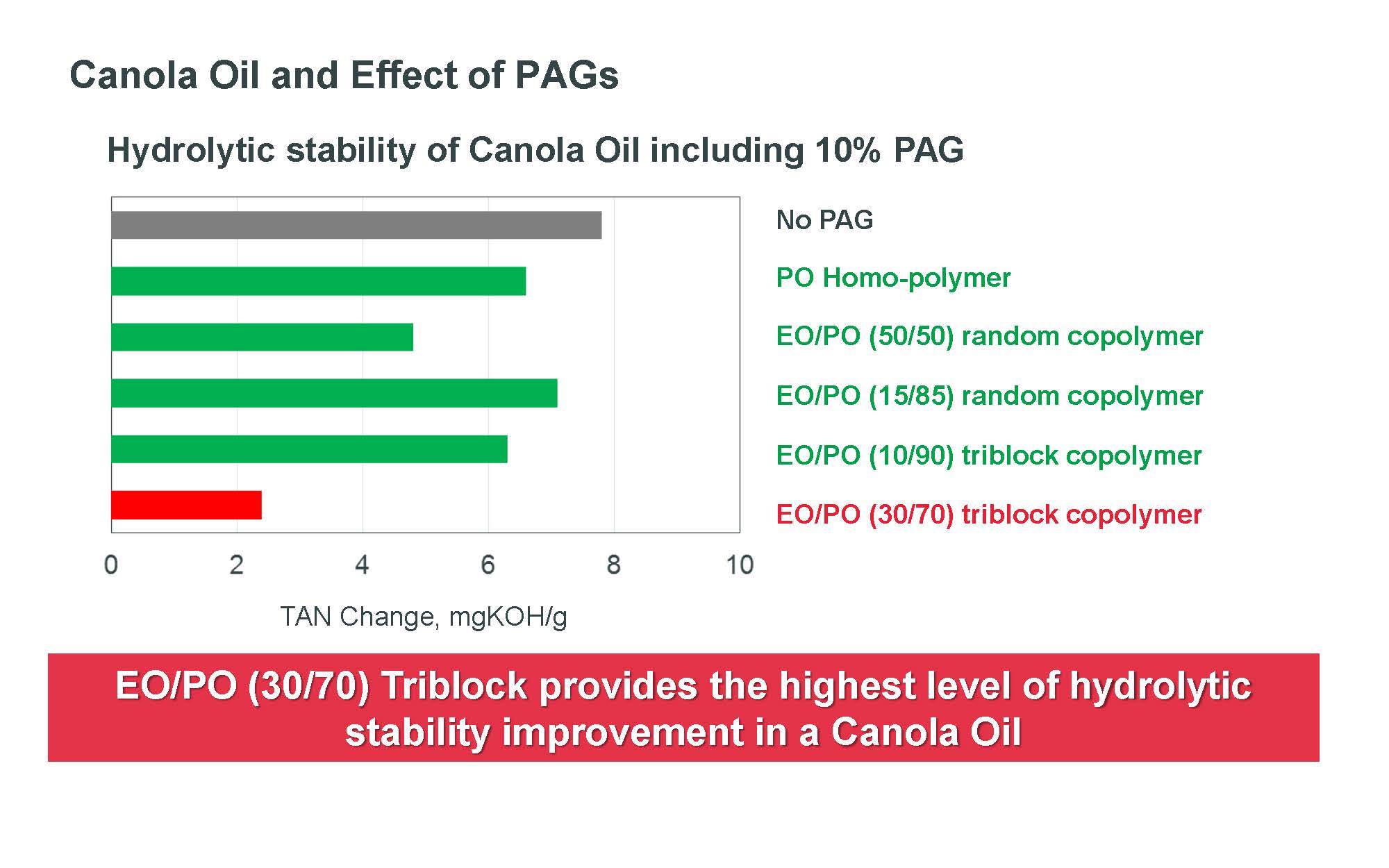 Figure 1. A triblock PAG copolymer with an ethylene oxide (EO) to propylene oxide (PO) ration of 30:70 demonstrates the lowest increase in acid number, which means the best hydrolytic stability, when used at a 10% treat rate in canola oil in testing that uses a modified ASTM D2619 procedure. (Figure courtesy of The Dow Chemical Co.)
Figure 1. A triblock PAG copolymer with an ethylene oxide (EO) to propylene oxide (PO) ration of 30:70 demonstrates the lowest increase in acid number, which means the best hydrolytic stability, when used at a 10% treat rate in canola oil in testing that uses a modified ASTM D2619 procedure. (Figure courtesy of The Dow Chemical Co.)
The effectiveness of the triblock copolymer may be due, in part, to the unique rheology behavior of block PAG structures in the presence of water. For example, addition of water in random copolymers typically results in a viscosity decrease of the PAG, but some block structures show a significant viscosity increase. The oxyethylene block in the copolymer is believed to hydrate and swell via hydrogen bonds with water molecules.
Greaves says, “We believe the behavior of the triblock copolymers when exposed to water may enable them to act as polymeric sponges. This result makes the triblock copolymers candidates for trapping water in lubricant compositions. One other benefit is that the PAG triblock copolymers are readily biodegradable.”
Future work will assess the performance of the triblock copolymers over a longer period of time. Greaves says, “We also intend to evaluate the effectiveness of triblock copolymers in field trials in an effort to validate the practical aspects of this invention.”
Additional information can be found from a recent presentation given at the 2017 STLE Annual Meeting (
3) and by contacting Greaves at
mrgreaves@dow.com.
REFERENCES
1.
Canter, N. (2009), “Upgrading biodegradable lubricant performance,” TLT,
65 (6), pp. 14-15.
2.
Sniderman, D. (2017), “VGP 2013: Propelling marine lubricants,” TLT,
73 (5), pp. 32-40.
3.
Huffman, L. and Greaves, M., “Improving the Hydrolytic Stability of Natural & Synthetic Esters Using Polyalkylene Glycols,” Presented at the STLE Annual Meeting in Atlanta, Ga., on May 22, 2017.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.