Ice repellent surfaces
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2011
Experimental data shows how superhydrophobic surfaces protect against ice build-up to temperatures below 0 C.
KEY CONCEPTS
•
Research has been conducted to show how dynamic water droplets interact with superhydrophobic surfaces under low temperature conditions.
•
Water droplets continuously dripped onto a superhydrophobic surface bounced off before ice nucleation could occur.
•
When allowed to freeze on a superhydrobic surface, water droplets remain in a non-wetting state that can be more easily removed using heat.
Improving the ability of lubrication systems to deal with water contamination is most important because water can serve to reduce operating life by accelerating processes such as corrosion. One approach that continues to be examined is the development of superhydrophobic surfaces that exhibit superior water repellency.
A previous TLT article discussed work that confirmed the presence of small gas pockets near superhydrophobic surfaces (
1). Research was described that indicates these bubbles are present and contribute to water repellency.
Under cold temperature conditions, ice may also play a role in creating problems in lubricant systems. There are a number of ways to deice surfaces, but none of them are very effective.
Lidiya Mishchenko, a graduate student at Harvard University in Cambridge, Mass., says, “Most applications have been directed at deicing airplanes. Options such as antifreeze, chemicals and pneumatic devices have been tried and are simply not effective.” Some of these techniques such as heat are inefficient from an energy standpoint, while others such as salts are corrosive.
One other application discussed in this column is deicing airplane runways. A recent TLT article described a new biobased deicing fluid derived from C3-C5 polyols that was found to be as effective as the current potassium acetate technology but much less corrosive (
2).
Much research has been devoted to evaluating the performance of static water droplets on superhydrophobic surfaces. But not much work has been done to examine the interaction of dynamic water droplets with superhydrophobic surfaces, especially not under freezing conditions.
Work has now been done to show how superhydrophobic surfaces can provide effective protection against ice build-up to temperatures well below 0 C.
DYNAMIC WATER DROPLET BEHAVIOR
Mishchenko and other researchers working with Dr. Joanna Aizenberg, Amy Smith Berylson Professor of Material Science at the Harvard School of Engineering and Applied Sciences, have determined what happens when a stream of water droplets maintained at a number of different temperatures are applied to three different types of surfaces maintained at temperatures between -35 C and room temperature.
Benjamin Hatton, a post-doctoral researcher working with Aizenberg, says, “We are involved in working with engineered surfaces and surface chemistry. This type of project fit in very well with our capabilities. Our objective was to see if, even under freezing conditions, the use of superhydrophobic surfaces will reduce the contact time with moving water droplets, thereby minimizing the chances that ice nucleation will start.”
The researchers dripped water droplets from a 10-centimeter height onto a flat aluminum substrate that is a hydrophilic material, a smooth fluorinated silicon material that is hydrophobic and a fluorinated silicon superhydrophobic surface that is fabricated with various surface features such as bristles, blades, honeycombs and bricks.
Figure 3 shows the various surfaces and the fabricated geometries used in preparing the superhydrophobic material. Water maintained at a temperature of 0 C was applied to these surfaces at a flow rate of 0.06 milliliters per second. The surfaces were tilted to a 30-degree angle and kept at temperatures of -10 C.
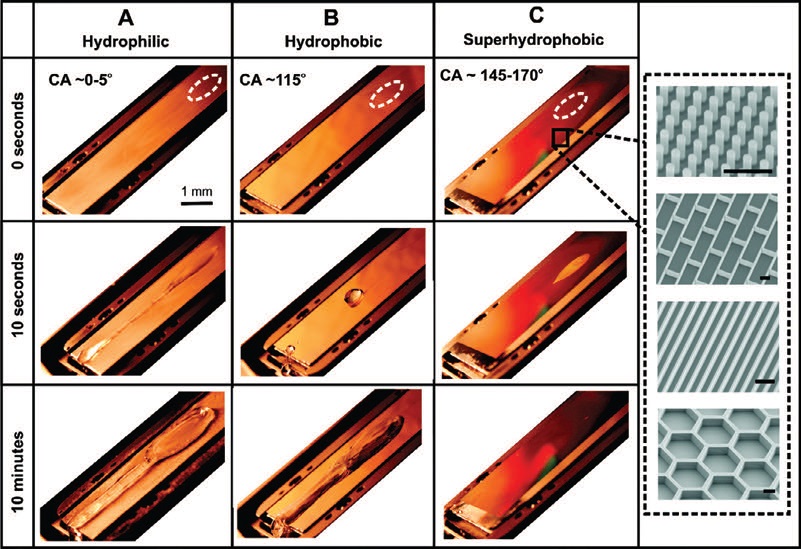 Figure 3. Water droplets were dripped onto aluminum (A – hydrophilic surface), fluorinated silicon (B – hydrophobic surface) and microstructured fluorinated silicon (c – superhydrophobic surface). no ice formation is seen on the superhydrophobic surface in contrast to the other two surfaces. (Courtesy of Harvard University)
Figure 3. Water droplets were dripped onto aluminum (A – hydrophilic surface), fluorinated silicon (B – hydrophobic surface) and microstructured fluorinated silicon (c – superhydrophobic surface). no ice formation is seen on the superhydrophobic surface in contrast to the other two surfaces. (Courtesy of Harvard University)
Mishchenko says, “We used an experimental setup with a high-speed camera to view the motion of the droplets onto the surfaces. The experimental setup is kept in a closed desiccant chamber and air is used to keep the environment relatively dry (humidity of approximately 5%) and reduce condensation of water on the substrates.”
Initial impact of the water droplets is noted with a dashed white line (
see Figure 3). The researchers detected ice formation on the hydrophilic and hydrophobic surfaces after the water droplets were continuously dripped onto these substrates for 10 minutes. For the superhydrophobic surface, no ice formation was detected, as seen in Figure 3.
To understand better the underlying principle of ice prevention on superhydrophobic surfaces, further work was conducted by dropping single droplets of water onto the three substrates held at temperatures below 0 C. in the case of the hydrophilic and hydrophobic surfaces, the droplet froze within seconds on both surfaces. The water droplet on the hydrophobic surface did retract into a spherical shape but still froze.
With the superhydrophobic surface, the droplet bounced off before ice nucleation could occur. When the water droplets do freeze on superhydrophobic surfaces at temperatures below -25 C, they can still be more easily removed than from smooth hydrophobic surfaces.
Mishchenko says, “On the superhydrophobic surface, we believe that the water droplet remains in a non-wetting state upon freezing. In this phenomenon, when the water droplet freezes on the surface it becomes stuck at the top of a surface feature and pinned in place. This droplet, when frozen upon impact, can thus be more easily removed through low levels of heat than a water droplet left on a smooth hydrophobic surface.”
Hatton summarizes the work that the researchers have done. He says, “Our study shows how controlling the kinetics of the ice nucleation process can be applied more widely. Limited surface area and time of contact enable water droplets to leave a surface and not form ice.”
The researchers intend to combine the extensive experimental work with a comprehensive theoretical model to provide guiding principles for developing materials that can be used on surfaces to minimize ice formation in a wide range of applications.
Use of superhydrophobic surfaces with fabricated geometries may prove to be useful in lubricant systems that are exposed to harsh low temperature conditions.
Further information can be found in a recent publication (
3) or by contacting Mishchenko at
lmishche@gmail.com.
REFERENCES
1.
Canter, N. (2010), “Presence of Nanobubbles on Superhydrophobic Surfaces,” TLT,
66 (8), pp. 14–15.
2.
Canter, N. (2010), “Runway Deicing Fluid is Environmentally Friendly,” TLT,
65 (1), pp. 14–15.
3.
Mishchenko, L., Hatton, B., Bahadur, V., Taylor, J., Krupenkin, T. and Aizenberg, J. (2010), “Design of Ice-free Nanostructured Surfaces Based on Repulsion of Impacting Water Droplets,”
ACS Nano,
4 (12), pp. 7699–7707.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.