•
A new cathodic material known as PTO and a new electrolyte that is a blend of solvents have been shown to boost the performance of a magnesium battery.
•
One reason for the strong performance is the low boron-to-magnesium ratio found in the PTO cathode.
•
The magnesium battery based on the new cathode and new electrolyte exhibited a power density that is two orders of magnitude higher than any previous magnesium battery.
Research is continuing to develop magnesium batteries with better power densities for potential use in electric vehicles. Magnesium batteries are under evaluation as an alternative to lithium-ion batteries. Anodes prepared from magnesium have been shown to exhibit much higher volumetric capacities and are potentially safer than lithium-ion batteries.
As noted in a previous TLT article,
1 a new design for a magnesium battery was prepared with an organic polymer-based cathode and a chloride-free electrolyte. The resulting battery displayed good stability when operated over 2,500 cycles with only a 13% drop in capacity observed.
Additional performance improvements are needed for magnesium batteries to emerge as potential alternatives to lithium-ion batteries. Yan Yao, Cullen professor of electrical and computer engineering at the University of Houston in Houston, Texas, says, “Magnesium ions move very slowly in a conventional intercalation cathode due to their divalent charge. One approach to improve cycling rate is to elevate the temperature of the operating battery. If commercialized, this strategy would require placement of a heater in a magnesium battery-powered electric vehicle. The higher vehicle weight reduces efficiency, making this approach impractical.”
A second problem is the presence of partially complexed ions such as the magnesium chloride cation in the magnesium electrolyte. Yao says, “Magnesium ions do not strictly cycle back and forth between the electrodes. Complexes such as the magnesium chloride cation are present in the electrolyte and are stored in the electrodes. Their presence results in a greater volume change of the cathode during charge-discharge cycles, leading to reduced cycle life and potentially causing mechanical failure.”
These observations indicate that a need exists for new cathodic materials to improve battery performance. Current electrolytes used in magnesium batteries also need to be upgraded. The previous TLT article on magnesium batteries highlighted the move to a chloride-free electrolyte based on boron cluster-based electrolyte known as carborane. Yao says, “Determining that carborane was a suitable electrolyte in combination with the organic solvent, tetraglyme was a big step forward, as it removed the need to use chloride-based electrolytes and reduced the possibility of corrosion.”
Yao says, “The problem with tetraglyme is the solvent’s high viscosity and tendency to coordinate with magnesium ions. Both factors limit mass transport across the battery reducing performance.”
Yao and his collaborators, including researchers at the Toyota Research Institute of North America, have now improved the performance of magnesium batteries by identifying a new cathodic material and modifying the boron-cluster-based electrolyte.
PTO
The researchers decided to work with PTO (pyrene-4,5,9,10-tetraone) as a new cathodic material because of its high capacity and compatibility with the boron-cluster-based electrolyte. Yao says, “We had previously worked with PTO in aqueous batteries and determined that this structure will produce a high discharge capacity due to the presence of four carbonyl groups in the molecule. During discharging, four electrons participated in the reaction through an enolization process that leads to reduction of the carbonyl species. An important factor is that the process is reversible during charging.”
Initial work demonstrated a high specific capacity and average discharge voltage. One reason was that inductively coupled plasma-optical emission spectroscopy (ICP-OES) analysis indicated that the ratio of boron to magnesium in the PTO cathode was 2.12. This result is in contrast to magnesium carborane, which has a boron to magnesium ratio of 11:1.
Yao says, “The analysis demonstrated that only a very small concentration of complex ions was present in the PTO cathode. Magnesium ions are the main species, leading to the high performance potential of PTO.”
One concern with the reduction process is that PTO will form an intermediate with magnesium that is soluble in the electrolyte. Fortunately, the final magnesium-PTO species is not soluble. Yao says, “Minimizing the presence of the soluble intermediate is needed to ensure that the cathodic material does not get lost in the electrolyte and can be recovered. This phenomenon was seen with magnesium-sulfur batteries where the problem is worse because the species, magnesium polysulfide, formed during reduction is much more soluble in tetraglyme.”
The researchers minimized the problem through the use of a thin (approximately two micron) and lightweight, graphene oxide membrane. The carboxyl groups present in graphene oxide act as ion-hopping sites for magnesium ions and reject negatively charged, PTO species. This membrane also acts as a physical barrier to prevent migration of the soluble magnesium PTO species.
A new electrolyte was found through evaluation of a series of solvent blends that involved mixing diglyme and a series of ether-based solvents such as tetrahydrofuran (THF), dimethyl ether (DME) and 1,3-dioxolane (DOL). The optimum blend ratio for these solvents was 1:1 with carborane. The best combination that reduced viscosity and minimized coordination of magnesium salts was carborane with a combination of diglyme and DME.
Adding the PTO cathode, new electrolyte and the graphene oxide membrane to a magnesium battery produced a power density that is two orders of magnitude higher than any previous magnesium battery. High stability was achieved with the battery showing 82% capacity retention after operating for over 200 cycles.
Figure 3 shows a schematic of the new battery that has PTO as the cathode, magnesium as the anode and a graphene oxide membrane present in the electrolyte between the electrodes.
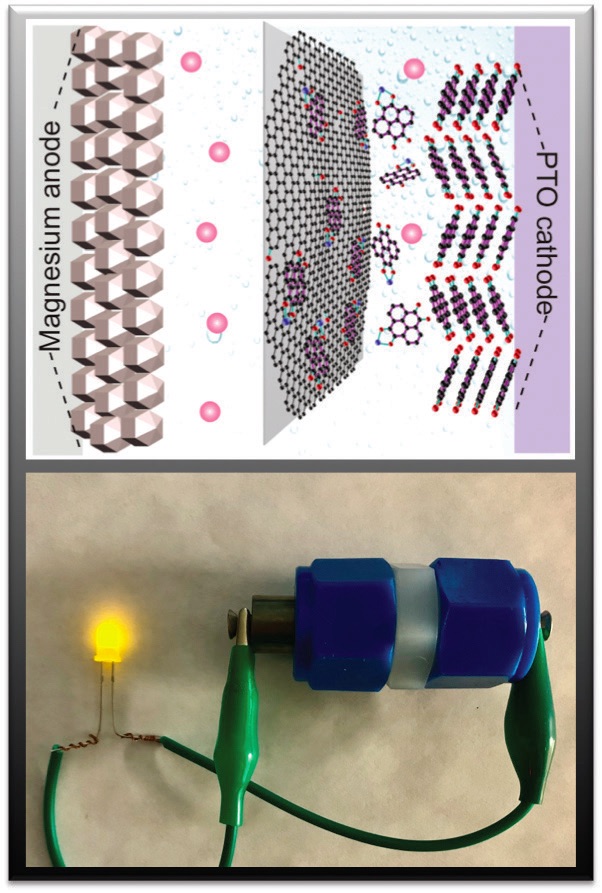 Figure 3. A schematic of a new magnesium battery containing a PTO cathode, a new electrolyte and a graphene oxide membrane is shown in the top image while the actual battery is shown in the bottom image. Figure courtesy of the University of Houston.
Figure 3. A schematic of a new magnesium battery containing a PTO cathode, a new electrolyte and a graphene oxide membrane is shown in the top image while the actual battery is shown in the bottom image. Figure courtesy of the University of Houston.
Yao says, “This research shows the potential of developing a magnesium battery that displays comparable power density to a lithium-ion battery when used at room temperature. Our next objective is to better understand the reaction pathway for how the soluble magnesium-PTO species forms during reduction and to reduce the percentage of carbon present in the cathode to increase cell-level energy density.”
Additional information can be found in a recent article
2 or by contacting Yao at
yyao4@uh.edu.
REFERENCES
1.
Canter, N. (2019), “New type of magnesium battery,” TLT,
75 (4), pp. 16- 17.
2.
Dong, H., Tutusaus, O., Liang, Y., Zhang, Y., Higgins, Z., Yang, W., Mohtadi, R. and Yao, Y. (2020), “High-power Mg batteries enabled by heterogeneous enolization redox chemistry and weakly coordinating electrolytes,”
Nature Energy, 5, pp. 1043-1050.