High-temperature fuel cells have advantages over low-temperature fuel cells because they can use lower purity hydrogen and are a much simpler system.
An ionic binder material known as PWN70 and based on a phosphonated polymer has been shown to enhance the performance of the catalyst used in a high-temperature membrane fuel cell.
Power densities of 1,130 milliwatts per square centimeter at 160 C under hydrogen/oxygen conditions were achieved with PWN70.
The movement to a hydrogen-based economy is accelerating the development of fuel cells as an efficient energy conversion device that generates electricity with an environmentally friendly byproduct: water. Fuel cells operate using a proton exchange membrane that conducts protons from the anode to the cathode.
Currently, proton exchange membrane fuel cells operate below 100 C to hydrate the membrane for proton conductions. In a previous TLT article,
1 researchers developed advanced fuel cells by replacing the platinum catalyst with tungsten carbide nanoparticles. This approach significantly improved the performance of the fuel cell under low humidity conditions by 80%. Better fuel cell durability also was achieved at 90 C because tungsten carbide more readily binds radical species that can form during fuel cell operation.
Research also is underway to optimize the performance of high-temperature membrane fuel cells that are designed to function at temperatures above 100 C. One area of improvement/optimization that is ongoing is described by Dr. Yu-Seung Kim, project leader at Los Alamos National Laboratory in Los Alamos, N.M., who says, “Low-temperature fuel cells face operational problems in powering automobiles. The fuel cell’s reaction that generates electricity is an exothermic, electrochemical process that causes the fuel cell’s temperature to increase. Placing stacks of fuel cells on top of each other in an engine places great demands on removing the excess heat generated. A large engine radiator might be required to maintain the temperature below 100 C. Operating fuel cells with large size radiator and low operating temperature adds cost and reduces power without leaving much space for the fuel cell stack.”
A second problem with using low-temperature fuel cells is their inability to be used to reformate hydrogen from natural gas and other clean liquid fuels such as methanol. Kim says, “Natural gas and other sources of hydrogen contain impurities that can poison the catalyst in the proton exchange membrane, which limits fuel cell performance. This means that low-temperature fuel cells need to rely directly on high-purity hydrogen from water electrolyzers. Distribution of compressed hydrogen also is challenging, and the compressed hydrogen storage still takes up much space in fuel-cell vehicles.”
High-temperature fuel cells can operate with low-grade reformate hydrogen because catalyst poisoning is minimal when the operating temperature is high. But this type of fuel cell has performance limitations that need to be overcome.
Kim says, “High-temperature fuel cells represent a much simpler system as large radiators and hydrogen tanks might not be needed. But the conductivity of protons across the membrane is very low at high temperature operating conditions, which limits fuel cell performance.”
Current high-temperature membrane fuel cells are based on the use of phosphoric acid-doped polybenzimidazole, which functions at high temperatures (140 C-180 C). Kim says, “This type of fuel cell can only be used for stationary applications. For automobile applications, loss of phosphoric acid at low temperature is problematic. To overcome this issue, we developed ion-pair coordinated membranes in 2016 that are based on biphosphate and ammonium coordination. But fuel cells using this membrane suffered from poor performance because of the need for a high-performance polymer electrolyte binder at the electrodes. Ion-pair coordinated membranes work well as membranes but cannot be used as electrode binders because of low gas permeability.”
A new polymer electrolyte material to improve the performance of high-temperature membrane fuel cells has now been developed.
Phosphonated polymers
Kim and his colleagues have developed a phosphonated polymer prepared from a tetrafluorinated styrene monomer that contains a phosphonic acid pendant group. The polymer is known as poly (2,3,5,6-tetra- fluorostyrene-4-phosphonic acid) and has a 70% degree of phosphonation. It is known as PWN70.
Kim says, “Our objective is to identify an ionomeric binder material that can be placed on the membrane to enhance the performance of the catalyst. Phosphonated polymers were looked at in the past because of their ability to conduct protons under hydrated and anhydrous conditions. But they were limited by poor mechanical properties and the tendency to form phosphonic acid anhydride during operation above 100 C.”
Phosphonic acid anhydrides were found to exhibit very low conductivity, hindering the performance of the fuel cell. The researchers determined that anhydride formation becomes favorable from a thermodynamic standpoint at a temperature of 160 C. It is an exergonic process.
The reason for designing PWN70 is to minimize anhydride formation under conditions experienced in a high-temperature membrane fuel cell. Kim says, “Anhydride formation takes place between the hydroxyl and hydrogen functionalities bonded to the phosphorus atom. By using a fluorinated polymer, the electron withdrawing properties hindered the activity of hydroxyl groups. This characteristic inhibited phosphonic acid anhydride formation.”
PWN70 became part of an ion-pair coordinated membrane in the membrane electrode assembly. Kim says, “We believe PWN70 is a very stable polymer due to covalent bonding that eliminates any possibility of acid loss. The hydrophobic nature of the fluorinated polymer facilitates the removal of water formed during fuel cell operation, further improving reactant gas access. Therefore, we can obtain good fuel cell performance with this material.”
Figure 1 shows an image of a membrane electrode assembly (black part) covered by a gasket.
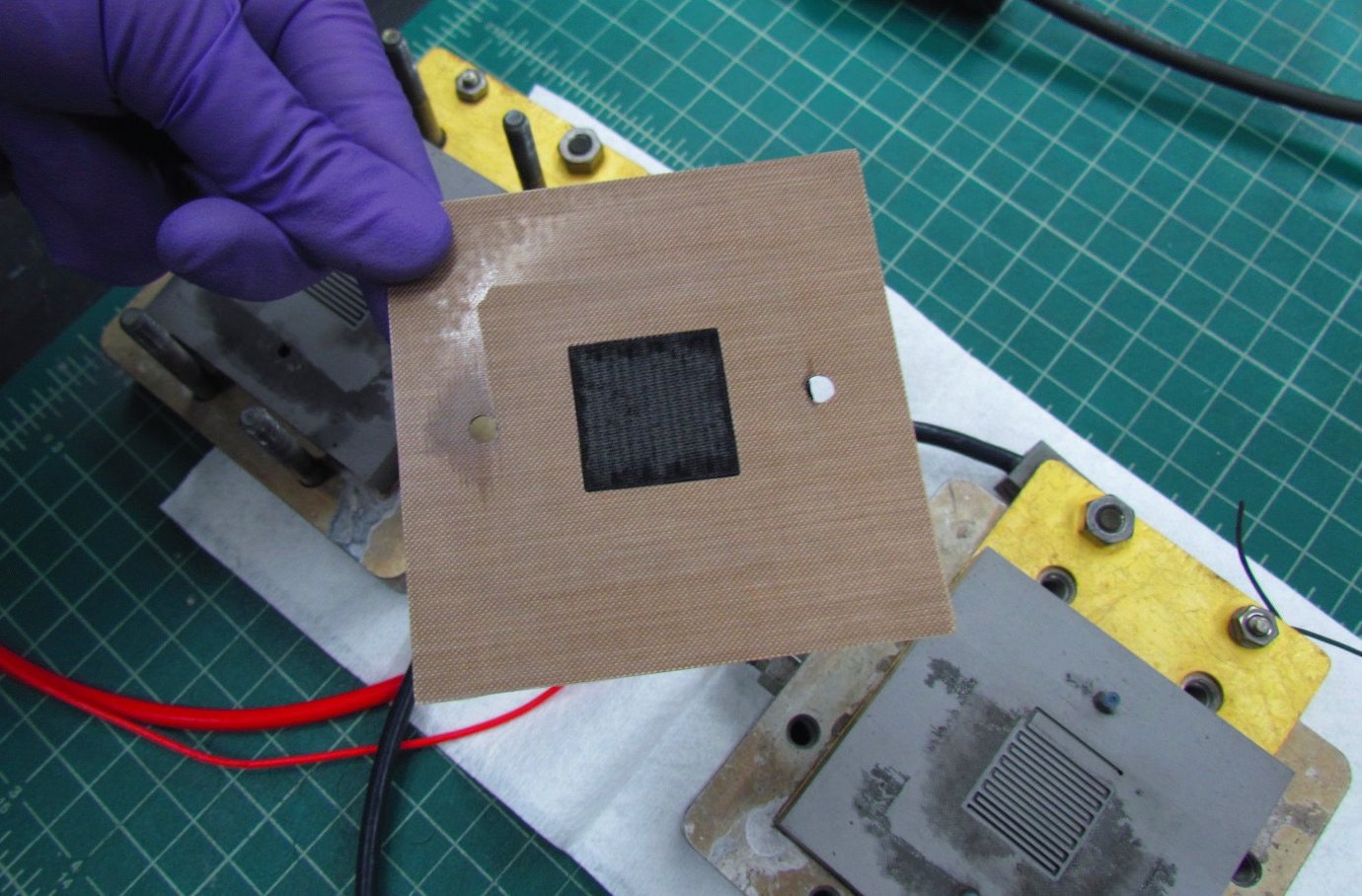 Figure 1. A high-temperature membrane electrode assembly (black part) covered by a gasket is shown in the foreground. The background shows a single high-temperature membrane fuel cell. Figure courtesy of Los Alamos National Laboratory.
Figure 1. A high-temperature membrane electrode assembly (black part) covered by a gasket is shown in the foreground. The background shows a single high-temperature membrane fuel cell. Figure courtesy of Los Alamos National Laboratory.
In testing against other membrane materials and electrode binders, PWN70 exhibited peak power densities of 1,130 milliwatts per square centimeter at 160 C under hydrogen/oxygen conditions and stable operation in the presence of water. This represents a much higher level of performance and water tolerance than was seen with previous polybenzimidazole-based fuel cells.”
Kim says, “We now have a clearer path to commercializing high-temperature membrane fuel cells. Future work will entail improving their efficiency, which means raising the current density at high cell voltage. Our objective is to develop a high-temperature membrane fuel cell with a power density above 1 watt per square centimeter under hydrogen/air conditions.”
Additional information can be found in a recent article
2 or by contacting Kim at
yskim@lanl.gov.
REFERENCES
1.
Canter, N. (2017), “More durable and cost-effective fuel cell,” TLT,
73 (12), pp. 12-13.
2.
Atanasov, V., Lee, A., Park, E., Maurya, S., Baca, E., Fujimoto, C., Hibbs, M., Matanovic, I., Kerres, J. and Kim, Y. (2020), “Synergistically integrated phosphonated poly(pentafluorostyrene) for fuel cells,”
Nature Materials, https://doi.org/10.1038/s41563-020-00841-z.